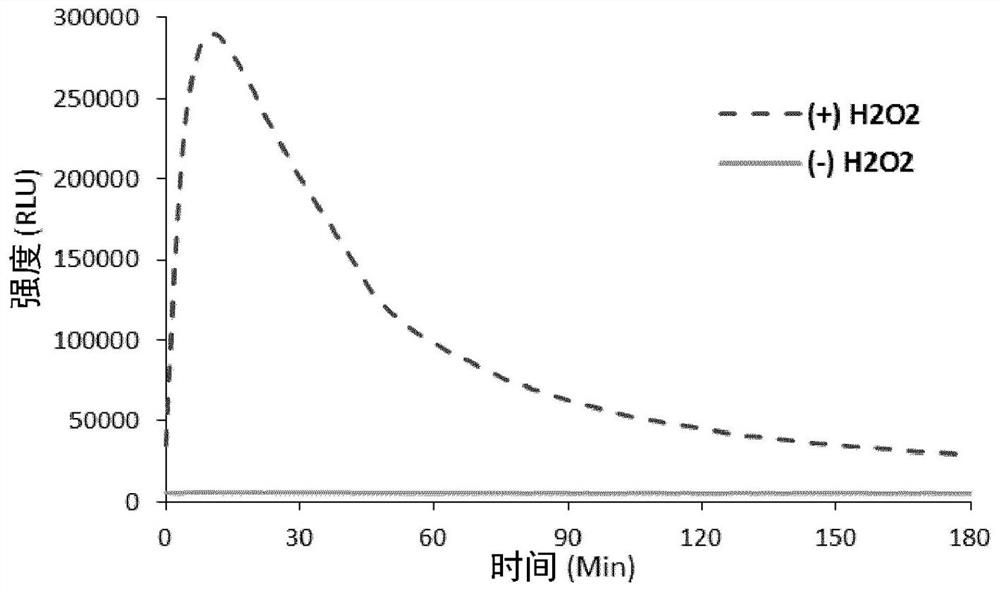
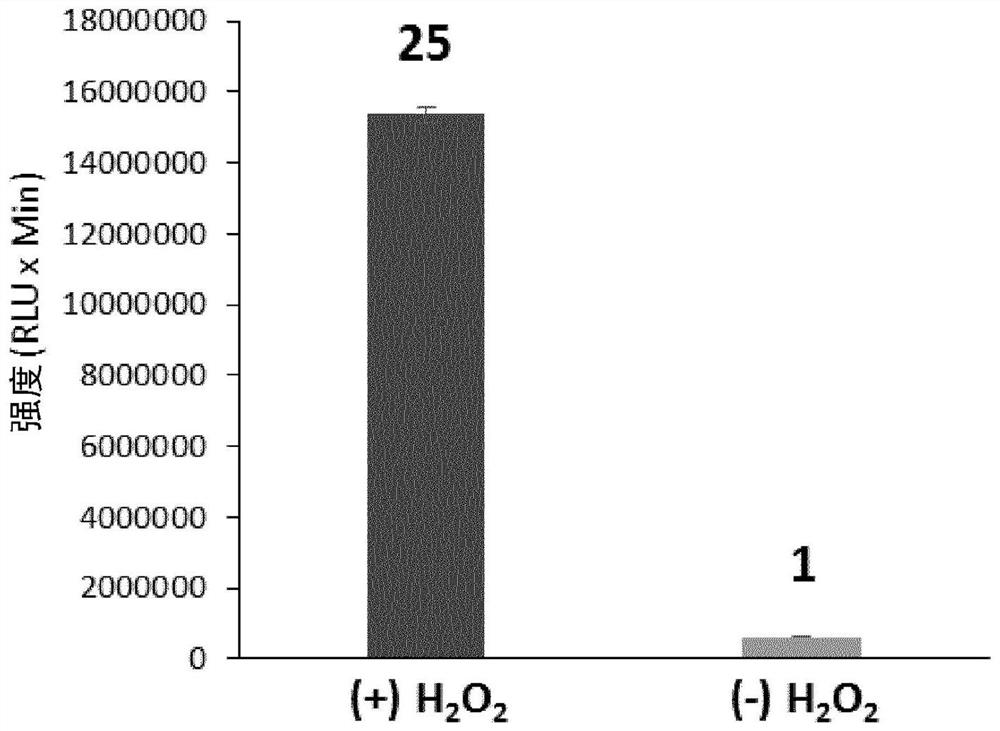
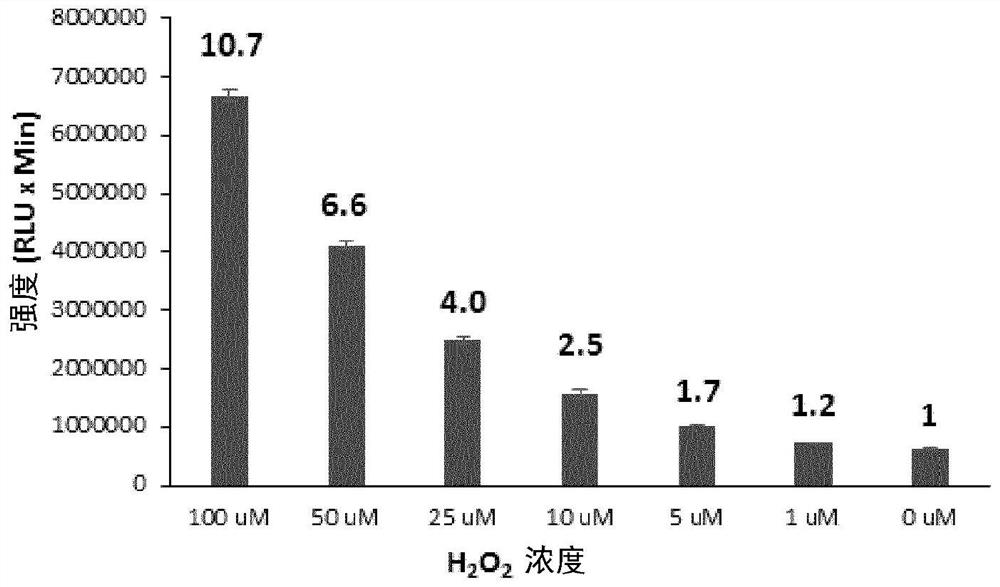
Long wavelength emitting chemiluminescent probes
A compound, straight-chain technology, applied in the compound of Ib and II, in the field of formula Ia, can solve the problems of complex synthesis, easy solubility, trouble and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0433] General method:
[0434] All reactions were performed at room temperature unless otherwise stated. Chemicals and solvents were A.R. grade or purified by standard techniques. Thin Layer Chromatography (TLC): Silica gel plates Merck 60F254: Compounds were visualized by irradiation with UV light. Column chromatography (FC): silica gel Merck 60 (particle size 0.040-0.063 mm), eluents are given in parentheses. Reverse-phase high-pressure liquid chromatography (RP-HPLC): C185μ, 250×4.6 mm, eluents are given in parentheses. Preparative RP-HPLC: C18 5μ, 250 x 21 mm, eluents are given in parentheses. Fluorescence and chemiluminescence were recorded on a Molecular Devices Spectramax i3x.
[0435] If not stated otherwise, all chemicals were purchased from Merck and Biosynth AG and used as received.
[0436] Abbreviation. AcOH-acetic acid, MeCN-acetonitrile, DCM-dichloromethane, DMF-N,N'-dimethylformamide, EtOAc-ethyl acetate, Hex-hexane, MeOH-methanol, TFA-trifluoroacetic aci...
Synthetic example 1
[0437] Synthesis Example 1: Synthesis of Exemplary Compounds
[0438] Synthesis was performed according to the following general scheme:
[0439]
[0440]
[0441]Aldehyde 1 (as described in "Chemiluminescent Probes for Activity-Based Sensing of Formaldehyde Released from Folate Degradation in Living Mice" (Angew. Chem. Int. Ed., 2018, Volume 130, Issue 25, Pages 7630-7634) was prepared; see Supplementary Information) (0.66 mmol, 220 mg) was dissolved in DMF (6.6 mL) and the solution was cooled to 0°C. Then add K 2 CO 3 (1.3eq., 0.86mmol, 120mg) and the reaction mixture was stirred at room temperature. Add iodide 2 (cf., Karton-Lifshin N., Albertazzi L., Bendikov M., Baran PS., Shabat D., J Am Chem Soc., 2012, 134(50), 20412-20) (1.3eq. , 0.86mmol, 296mg) and the reaction mixture was stirred at room temperature for 3h. By TLC (Hex / Et 2 O=9:1) to monitor the reaction. After completion, the mixture was cooled to 0 °C and washed with Et 2 Dilute with O (15 mL) and...
Synthetic example 2
[0448] Synthesis Example 2: Synthesis of Exemplary Compounds
[0449] Synthesis was performed according to the following general scheme:
[0450]
[0451] Compound 1 (1.5 mmol, 0.50 g) and 4-picoline (2) (1.53 mmol, 0.143 g) were placed in acetic anhydride and the mixture was stirred under reflux. After 6.5 h, the mixture was cooled to room temperature, MeOH (10 mL) and K 2 CO 3 And the resulting mixture was stirred at room temperature. After 2 h, the mixture was concentrated in vacuo and saturated NH 4 Aqueous Cl solution (20 mL). The resulting mixture was extracted with EtOAc (3 times, 20 mL), the combined organics were dried and concentrated in vacuo. The crude mixture was purified by column chromatography to yield compound 3 (0.256 g, 42%) as a yellow solid.
[0452] 1 H NMR (300MHz, CDCl 3 )δppm 1.20-2.40 (m, 13H), 3.28 (bs, 1H), 3.33 (s, 3H), 6.88 (d, J = 8.0Hz, 1H), 7.14 (d, J = 16.5Hz, 1H), 7.40 (bd, J=5.4Hz, 2H), 7.47 (dd, J=8.0Hz, 0.4Hz, 1H), 7.62 (d, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com