Chemiluminescent probe for detecting fibroblast activation protein, its synthesis method and application
A fibroblast, chemiluminescence technology, applied in chemical instruments and methods, chemiluminescence/bioluminescence, analysis by chemical reaction of materials, etc., to achieve the effect of expanding the scope of application and important application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
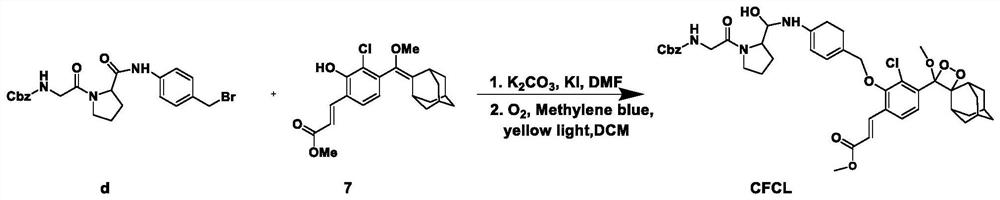
[0041] Example 1: Synthesis of the adamantane-dioxetane chemiluminescent probe CFCL for the detection of fibroblast activation protein (see the appendix for the synthesis route). figure 1 shown).
[0042] (1) Synthesis of compound 7, the specific steps are:
[0043] (1) Dissolve m-hydroxybenzaldehyde (25.6 g, 209.6 mmol, 1 equiv.) in 150 mL of 90 % acetic acid, after the temperature of the reaction solution drops to 0 °C, add tert-butyl hypochlorite (25.0 g, 230.6 mmol, 1.1 equiv.); the reaction was monitored by high performance liquid chromatography; after the reaction was complete, the product (referred to as compound 1) was obtained by filtration as a white solid 13.8 g (42.2 % crude product yield); MS (ESI-): m / z C 7 H 5 ClO 2 Theoretical value: 155.00, 156.99; visible [M-H]-154.88, 156.88;
[0044] (2) Compound 1 (content based on 100%, 10.8 g, 69.2 mmol, 1 equiv.) was dissolved in 100 mL of methanol, and tetrabutylammonium tribromide (1.7 g, 3.5 mmol, 0.05 equiv.)...
Embodiment 2
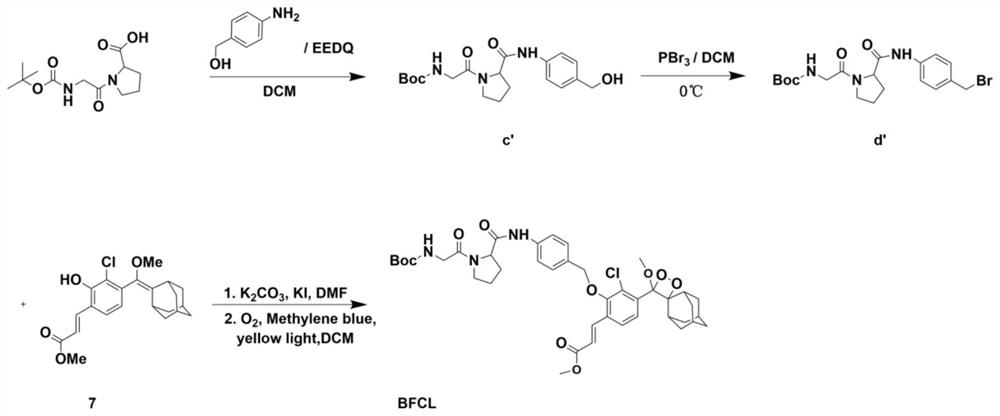
[0057] Example 2: Synthesis of adamantane-dioxetane chemiluminescent probe BFCL for detection of fibroblast activation protein. (See the synthetic route figure 2 shown), the specific steps are:
[0058] (1) Combine tert-butoxycarbonyl-glycine-proline-OH (1.1 g, 44.04 mmol, 1equiv.) and ethyl 2-ethoxy-1(2H)-quinolinecarboxylate (2.0 g, 8.08 mmol). , 2 equiv.) was dissolved in 20 mL of dichloromethane and a solution of p-aminobenzyl alcohol in dichloromethane (1.0 g, 8.08 mmol, 2 equiv.) was added dropwise. The reaction was monitored by high performance liquid chromatography. After the reaction, an appropriate amount of dichloromethane was added to dilute and washed with water. The organic phases were combined, and after the saturated sodium sulfate solid was dried, the solvent was removed by vacuum rotary evaporation, and purified by silica gel column chromatography. The obtained product (referred to as compound b1) was a white solid, totaling 1.24 g (82.7 % yield);
[005...
Embodiment 3
[0061] Example 3: CFCL or BFCL assay for fibroblast activation protein in vitro.
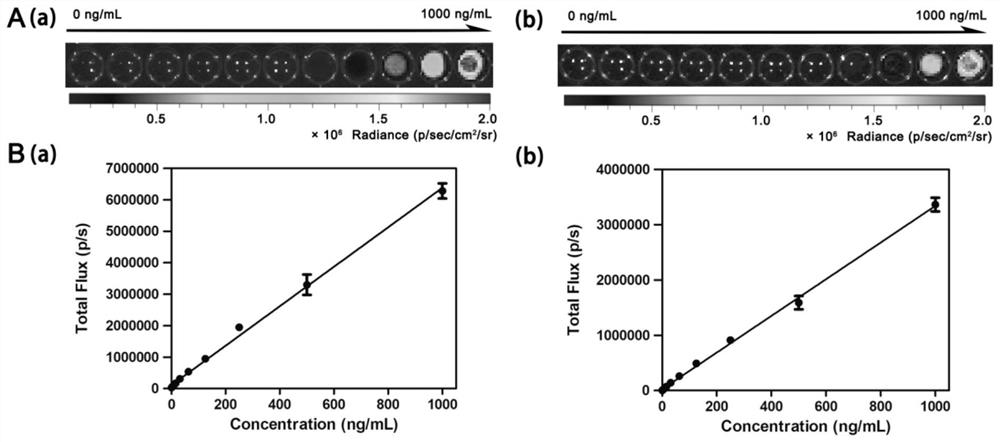
[0062] A series of FAPα solutions of different concentrations were prepared, and 100 μL of each concentration was placed in a small well of a black 96-well plate, and 100 μL of CFCL or BFCL chemiluminescence probe with a concentration of 40 μM was added to each well, which was obtained by Xenogen IVIS. ® The in vivo imager was photographed and quantitatively analyzed by the instrument's own software. like image 3 As shown, the response value increases with the increase of FAPα concentration, and there is a good linear relationship between the response value and FAPα concentration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com