Preparation method of tenofovir alafenamide semifumarate
A technology of tenofovir alafenamide and hemifumarate, which is applied in the field of single crystal preparation, can solve the problems of no single crystal in the cultivation of the compound, difficulty in cultivating single crystal, difficulty in single crystal cultivation, etc., and achieves the goal of preparing The conditions are easy to control, the solution is not easy to cultivate, and the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
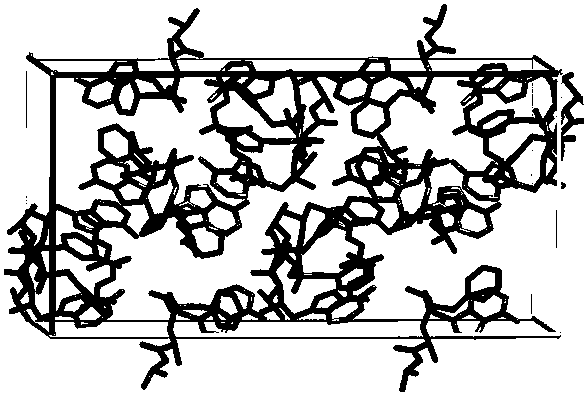
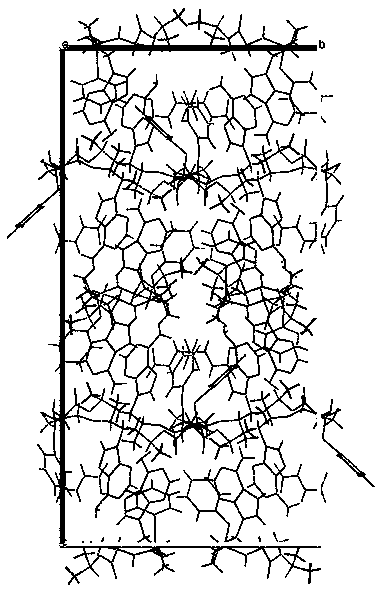
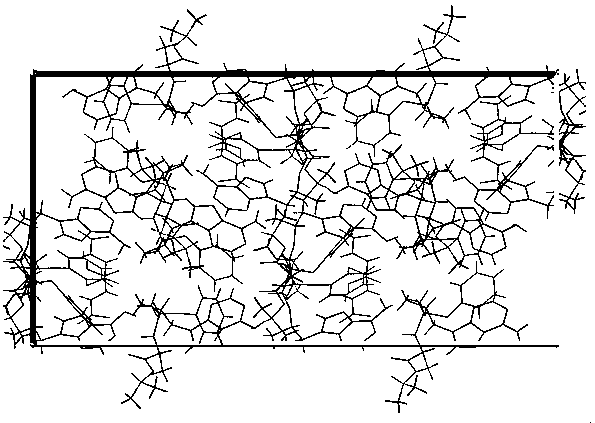
[0030] Dissolve tenofovir alafenamide and fumaric acid in 30 times the total mass of tenofovir alafenamide and fumaric acid in acetonitrile at room temperature at a molar ratio of 2:1, and the resulting mixture is placed in In the vial, the mouth of the bottle is partially closed so that the solvent can be volatilized, and then placed in an oven at 40°C, volatilized until a transparent cubic single crystal is obtained, and the colorless and transparent tenofovir alafenamide hemifumarate is obtained. Cubic single crystal.
Embodiment 2
[0032] Dissolve tenofovir alafenamide and fumaric acid in 30 times the total mass of tenofovir alafenamide and fumaric acid in acetonitrile at room temperature at a molar ratio of 2:1, and the resulting mixture is placed in In the vial, the mouth of the bottle is partially closed to make the solvent volatilize, and then placed in an oven at 45°C, volatilized until a transparent cubic single crystal is obtained, and a colorless transparent cube of tenofovir alafenamide hemifumarate is obtained single crystal.
Embodiment 3
[0034] Dissolve tenofovir alafenamide and fumaric acid in 30 times the total mass of tenofovir alafenamide and fumaric acid in acetonitrile at room temperature at a molar ratio of 2:1, and the resulting mixture is placed in In the vial, the mouth of the bottle is partially closed to make the solvent volatilize, and then placed in an oven at 55°C, volatilized until a transparent cubic single crystal is obtained, and a colorless transparent cube of tenofovir alafenamide hemifumarate is obtained single crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com