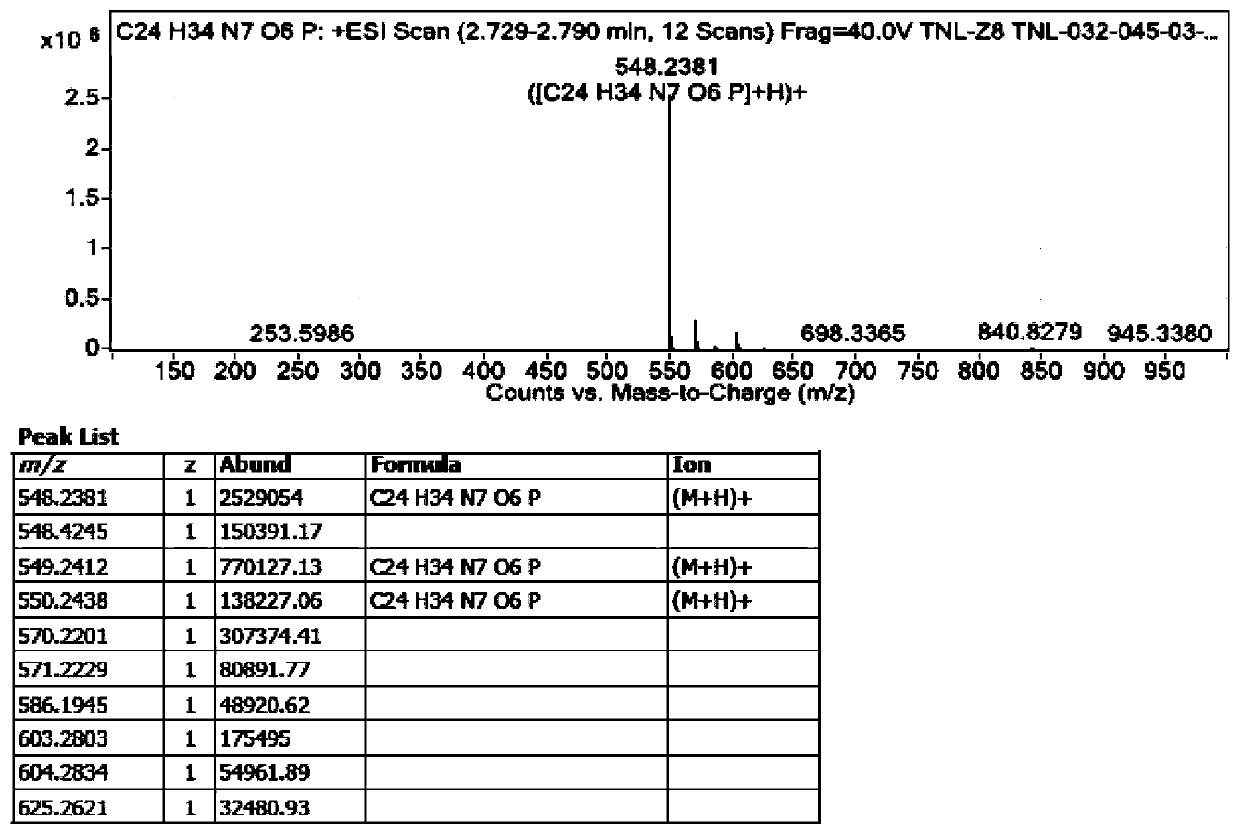
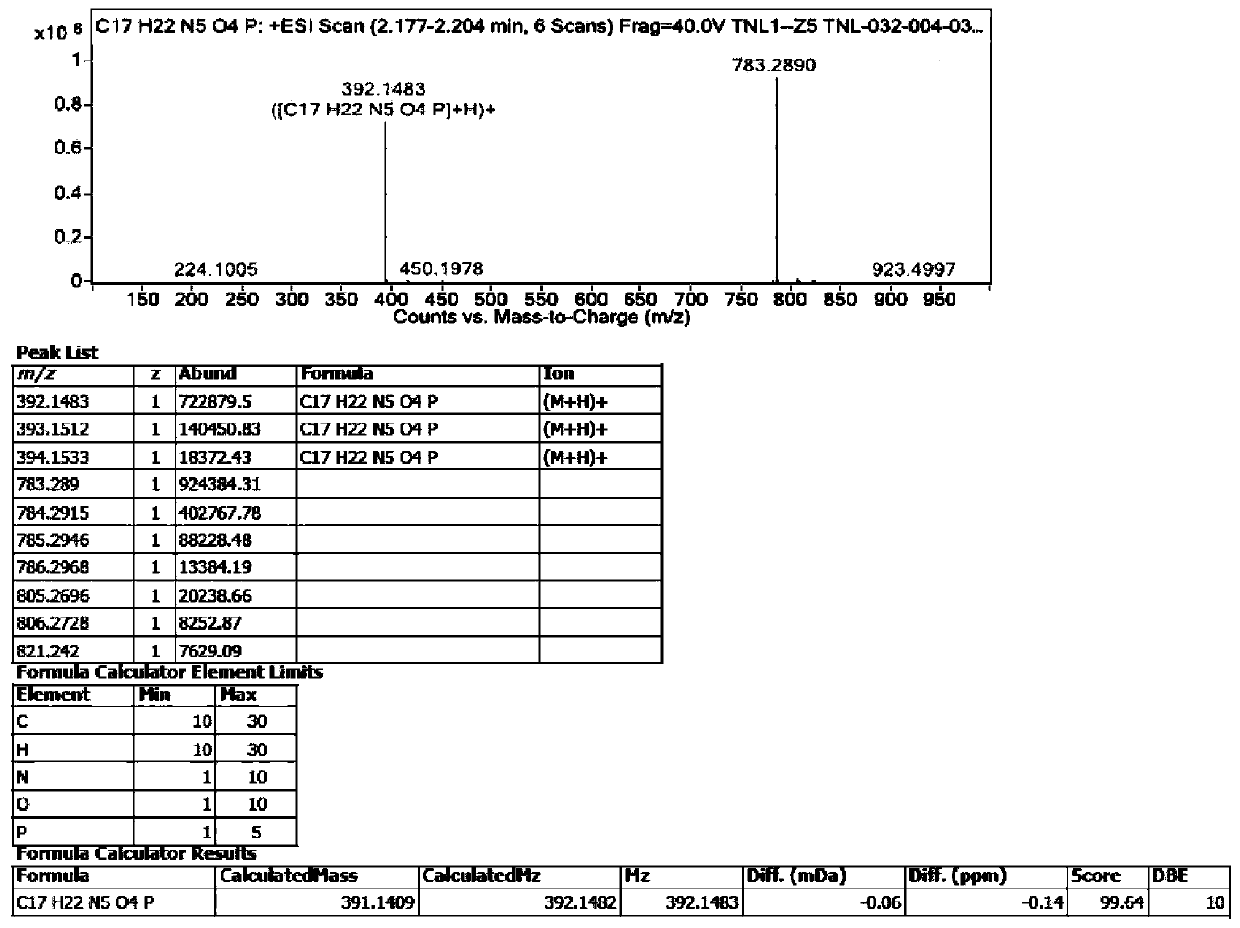
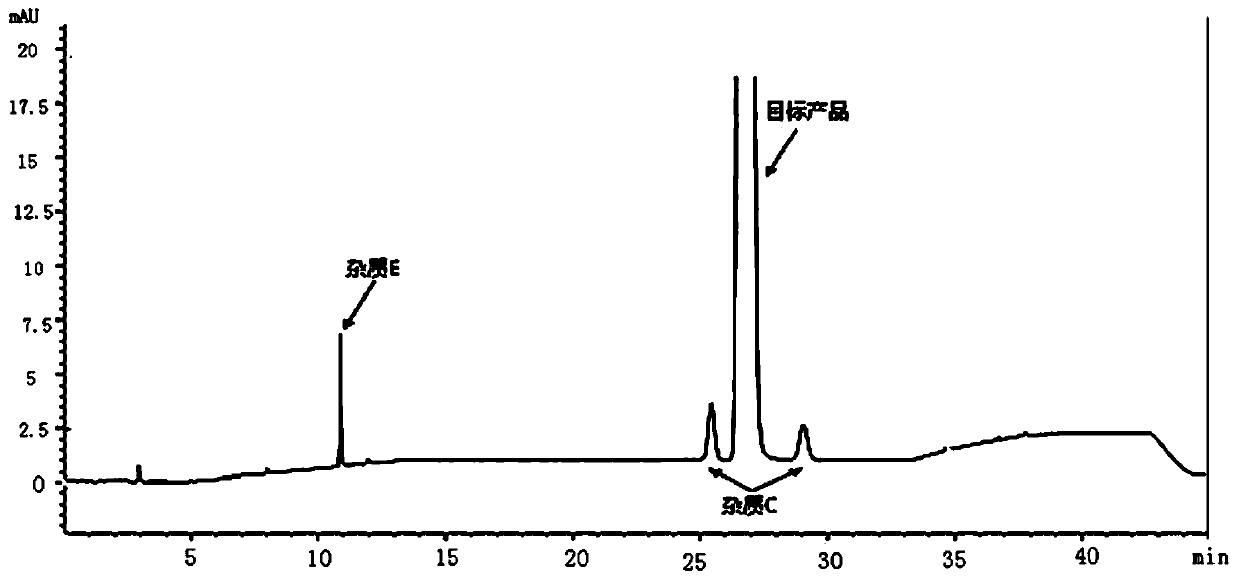
Tenofovir alafenamide series impurities and synthesis method thereof
A technology of tenofovir alafenamide and synthetic method, applied in the field of drug impurity synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0052] Preparation of Compound C:
[0053] Tenofovir monophenyl ester (compound I) 10.0g (27.55mmol, 1.0eq), acetonitrile 80g and thionyl chloride 6.6g (55.1mmol, 2.0eq) were added to the bottle, the temperature was raised to 80°C, and the reaction was stirred for 3.5 h, the reaction solution was concentrated to dryness, 40 g of dichloromethane was added to the system to dissolve, and 3.3 g of L-alanyl-L-alanine isopropyl ester (compound H) (16.53 In the solution of mmol, 0.6eq) and 10g dichloromethane, triethylamine is added dropwise at the same time to keep the pH at 6-8. The dichloromethane phase was washed three times with saturated sodium bicarbonate and sodium chloride respectively, the dichloromethane phase was concentrated, and isopropyl ether was added to precipitate a white solid, which was filtered and dried to obtain 2.14 g of a sample, which was compound C.
[0054] 1H NMR (400MHz, D2O): δ8.20-8.10(3H), 7.35-7.24(2H), 7.21(1H), 7.17-7.11(2H), 6.9(1H), 5.3(1H), 4....
Embodiment 1-2
[0056] Preparation of Compound C:
[0057]Add tenofovir monophenyl ester (compound I) 10.0g (27.55mmol, 1.0eq), acetonitrile 80g and thionyl chloride 3.3g (27.55mmol, 1.0eq) into the bottle, heat up to 50°C, and stir for 2h , Concentrate the reaction solution to dryness, add 40g of dichloromethane to the system to dissolve, and add 1.1g of L-alanyl-L-alanine isopropyl ester (compound H) (5.51 mmol, 0.2eq) and 10g dichloromethane solution, dripping triethylamine simultaneously keeps the pH to be 6-8, stirs and reacts for 2 hours after dripping, adds dichloromethane 200g and water 100g extracting liquid separation in the system, The dichloromethane phase was washed three times with saturated sodium bicarbonate and sodium chloride respectively, the dichloromethane phase was concentrated, and purified by column chromatography to obtain 0.31 g of a sample, which was Compound C.
[0058] 1H NMR (400MHz, D2O): δ8.20-8.10(3H), 7.35-7.24(2H), 7.21(1H), 7.17-7.11(2H), 6.9(1H), 5.3(1H),...
Embodiment 1-3
[0062] Preparation of Compound C:
[0063] Add tenofovir monophenyl ester (Compound I) 10.0g (27.55mmol, 1.0eq), acetonitrile 80g and thionyl chloride 13.2g (110.2mmol, 4.0eq) into the bottle, raise the temperature to 70°C, and stir for 6h , the reaction solution was concentrated to dryness, 40 g of dichloromethane was added to the system to dissolve, and 5.5 g (27.55 mmol, 27.55 mmol, 1.0eq) and 10g of dichloromethane solution, drop triethylamine at the same time to keep the pH at 6-8, stir and react for 3 hours after dropping, add 200g of dichloromethane and 100g of water to the system for extraction and separation, DCM phase Wash with saturated sodium bicarbonate and sodium chloride three times respectively, concentrate the dichloromethane phase, and purify by column chromatography to obtain 2.32 g of the sample, which is compound C.
[0064] 1H NMR (400MHz, D2O): δ8.20-8.10(3H), 7.35-7.24(2H), 7.21(1H), 7.17-7.11(2H), 6.9(1H), 5.3(1H), 4.8(1H) , 4.30-4.25, 4.25-4.12 (2H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com