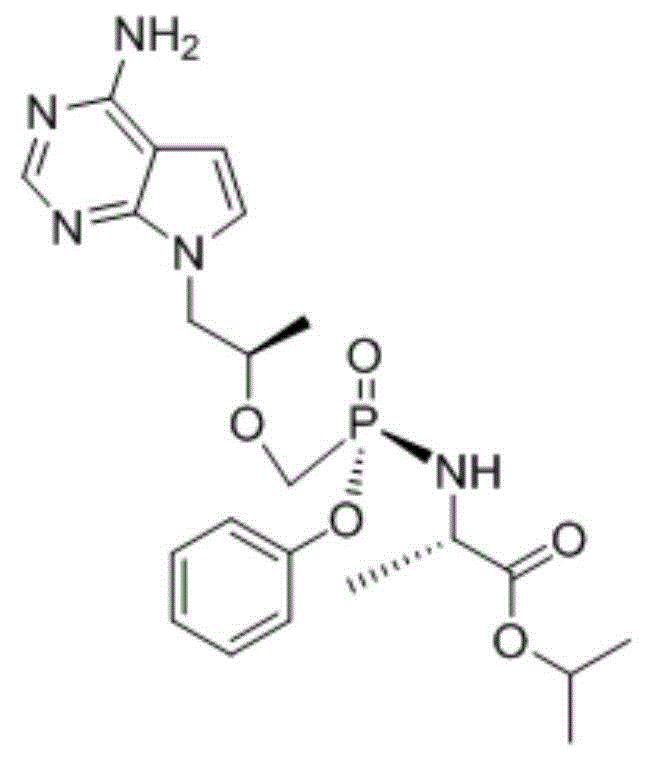
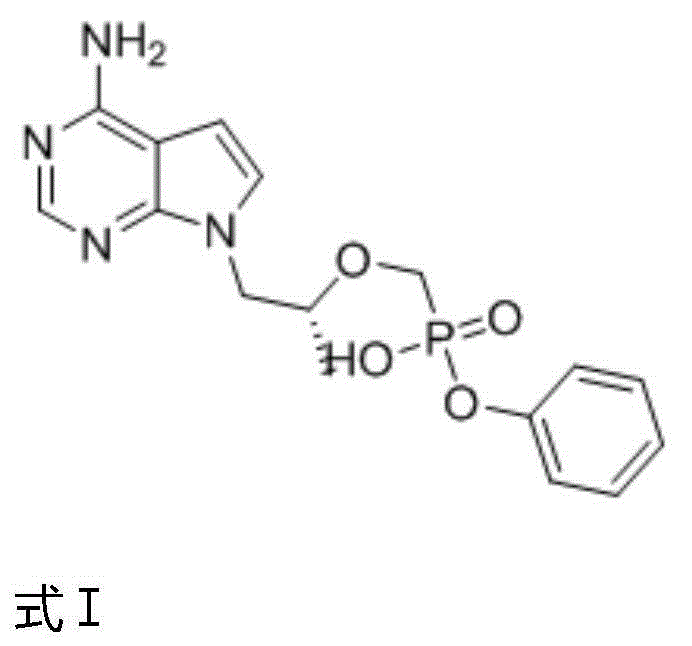
TAF(tenofovir alafenamide fumarate) preparation method
An adenine and reaction technology, applied in the field of preparation of TAF, can solve the problems of large pollution and post-treatment, affecting industrial production, etc., and achieve the effects of reducing pollution, optimizing reaction conditions, and optimizing reaction solvent.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add adenine to sodium hydroxide solution, then add ethylene carbonate, the molar ratio of adenine to ethylene carbonate is 1:1-1:1.5; the reaction temperature is 150°C, react for 24 hours, and then add ethyl acetate The ester was recrystallized and filtered to give the solid product 9-(2-hydroxyethyl)adenine.
Embodiment 2
[0034] Suspend 9-(2-hydroxyethyl)adenine in dimethylformamide, and add magnesium isopropoxide to it, wherein the amount of magnesium isopropoxide is 9-(2-hydroxyethyl)adenine One-half, then add p-toluenesulfonyloxymethylphosphonic acid diethyl ester, p-toluenesulfonyloxymethylphosphonic acid diethyl ester and 9-(2-hydroxyethyl) adenine The ratio is 1.5-2:1. The reaction temperature is 95° C., and the reaction time is 10 h. After the reaction is completed, 9-[2-(phosphorylmethoxy)ethyl]adenine is extracted and separated by acetone.
Embodiment 3
[0036] Add phenol and mercaptopropionic acid to 9-[2-(phosphorylmethoxy)ethyl]adenine, wherein the molar ratio of phenol to 9-[2-(phosphorylmethoxy)ethyl]adenine is 2-2.5:1, the molar ratio of mercaptopropionic acid to 9-[2-(phosphorylmethoxy)ethyl]adenine is 1.5-2:1. The reaction temperature is 120°C, and the reaction time is 20h. After the reaction was completed, it was extracted and separated with ether to obtain the intermediate shown in formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com