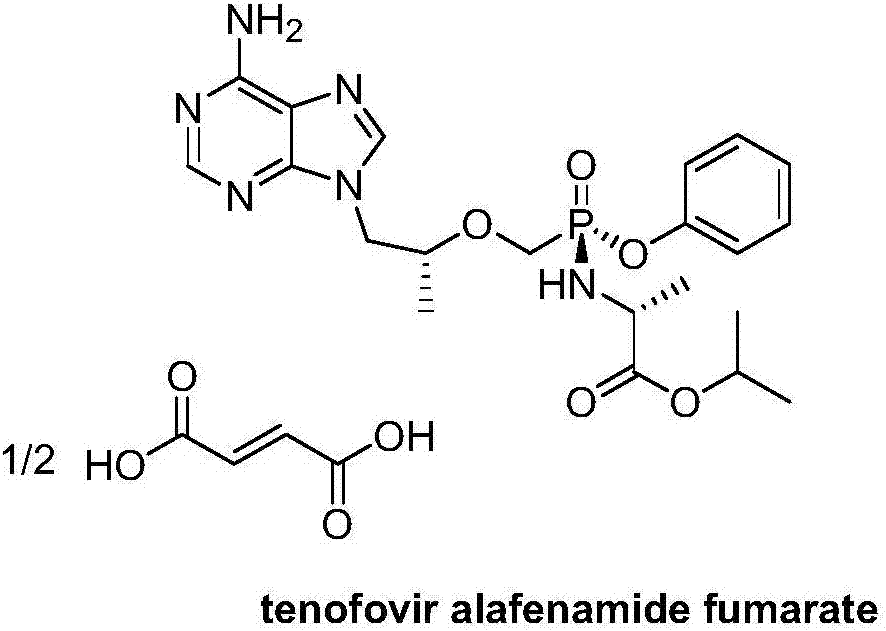
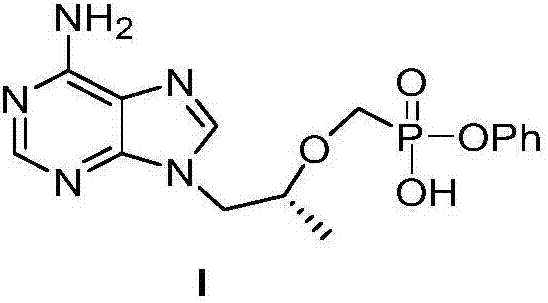
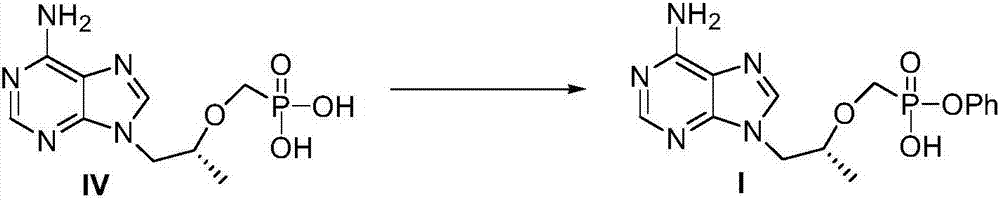
Preparation method of TAF (tenofovir alafenamide fumarate) nucleoside derivative and intermediate of TAF nucleoside derivative
A volume and compound technology, applied in the field of preparation of TAF nucleoside derivatives and its intermediates, can solve the problems of large environmental pollution, low yield, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 compound III
[0036]
[0037] Put IV (100g, 0.348mol) in a 2L three-neck flask, add thionyl chloride (1L) under nitrogen protection, heat to an external temperature of 70 degrees and reflux for overnight reaction. TLC shows that the reaction is complete, and the solvent is directly evaporated to dryness to obtain V The crude product of light yellow solid 110g (0.341mol, yield 97.9%).
[0038]
[0039] Put 100g (0.310mol) of the crude product of V in a 2L three-neck flask, add anhydrous acetonitrile (1L) under nitrogen protection, stir to dissolve and cool to 0°C, add phenol (116g, 1.23mol), slowly rise to 25°C Reacted for 16 hours, TLC showed that the reaction was complete, quenched the reaction with saturated sodium bicarbonate solution, extracted and separated layers with dichloromethane, washed the organic phase with saturated sodium chloride, dried over anhydrous magnesium sulfate, filtered and evaporated to dryness, and washed...
Embodiment 2
[0042] Embodiment 2R is methyl
[0043]
[0044] Put compound (R)-9-(2-diphenoxyphosphomethoxypropyl)adenine (III) 100g (0.228mol) in a 2L three-necked flask, add anhydrous acetonitrile (1L) under nitrogen protection , stirred to dissolve and cooled to 0 degrees, added methanol (20g), slowly rose to 25 degrees Celsius for 16 hours, TLC showed that the reaction was complete, saturated sodium bicarbonate solution quenched the reaction, dichloromethane extraction layered, organic phase with saturated chlorine Wash with sodium chloride, dry over anhydrous magnesium sulfate, filter and evaporate the solvent to obtain 83 g (0.220 mol, yield 96.6%) of the desired product II-1.
[0045] Characterization data NMR (400MHz,d 6 -DMSO):8.13(s,1H),8.11(s,1H),7.55(br,2H),7.25(m,2H),7.08(m,1H),7.03(m,2H),4.22(m, 2H), 3.95(br,1H), 3.75(m,3H), 1.02(br,3H).
Embodiment 3
[0046] The preparation of embodiment 3 compound I
[0047]
[0048] Put 83g (0.220mol) of II-1 in a 2L three-neck flask, add 1.35L of 1,4-dioxane, 1M potassium hydroxide aqueous solution (350mL, 0.35mol), stir at 25°C for 3 hours, TLC shows After the reaction is complete, add water (350mL) and extract twice with ethyl acetate (350mL). The aqueous phase is adjusted to pH=2-3 with concentrated hydrochloric acid, and solid I is precipitated. Filter, wash the filter cake with 2N hydrochloric acid, and dry it in an oven at 60 degrees. I was obtained (78 g, 0.215 mol, yield 97.7%).
[0049] Characterization data NMR (400MHz,d 6-DMSO):8.13(s,1H),8.11(s,1H), 7.55(br,2H),7.25(m,2H),7.08(m,1H),7.03(m,2H),4.22(m, 2H), 3.95(br,1H), 3.75(m,3H), 1.02(br,3H).
[0050] ESI-MS[M+H] + :364.14
[0051] Embodiment 4R is ethyl
[0052]
[0053] Put compound (R)-9-(2-diphenoxyphosphomethoxypropyl)adenine (III) 100g (0.228mol) in a 2L three-necked flask, add anhydrous acetonitrile (1L) u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com