Tenofovir alafenamide fumarate compound and pharmaceutical composition thereof
A technology of alafenamide fumarate compound and alafenamide fumarate is applied in the field of pharmaceutical compositions containing the tenofovir alafenamide fumarate compound, which can solve the problem of Issues such as crystal form and data are disclosed to achieve the effect of simple preparation method and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of Form I of tenofovir alafenamide fumarate
[0056] Method 1: Take 500 mg of tenofovir alafenamide fumarate, add 10 mL of methanol at room temperature, add dropwise 200 mL of isopropyl ether under stirring, a large amount of solid is precipitated, centrifugally filtered, and vacuum dried overnight at room temperature to obtain.
[0057] Method 2: Take 500 mg of tenofovir alafenamide fumarate, add 8 ml of methyl tert-butyl ether at room temperature, stir and crystallize, centrifuge and filter, and dry in vacuum at room temperature.
[0058] Method 3: Take 500mg of tenofovir alafenamide fumarate, add 15ml of tetrahydrofuran to obtain a solution, add the solution to 200ml of methyl isopropyl ether, crystallize, centrifugal filter, and vacuum dry overnight at room temperature to obtain .
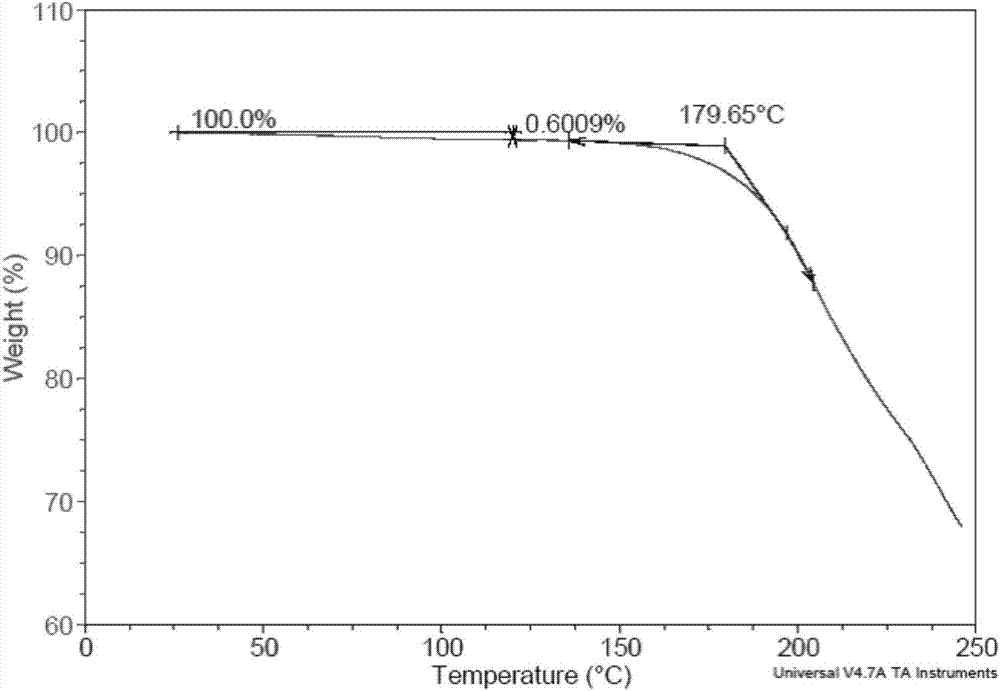
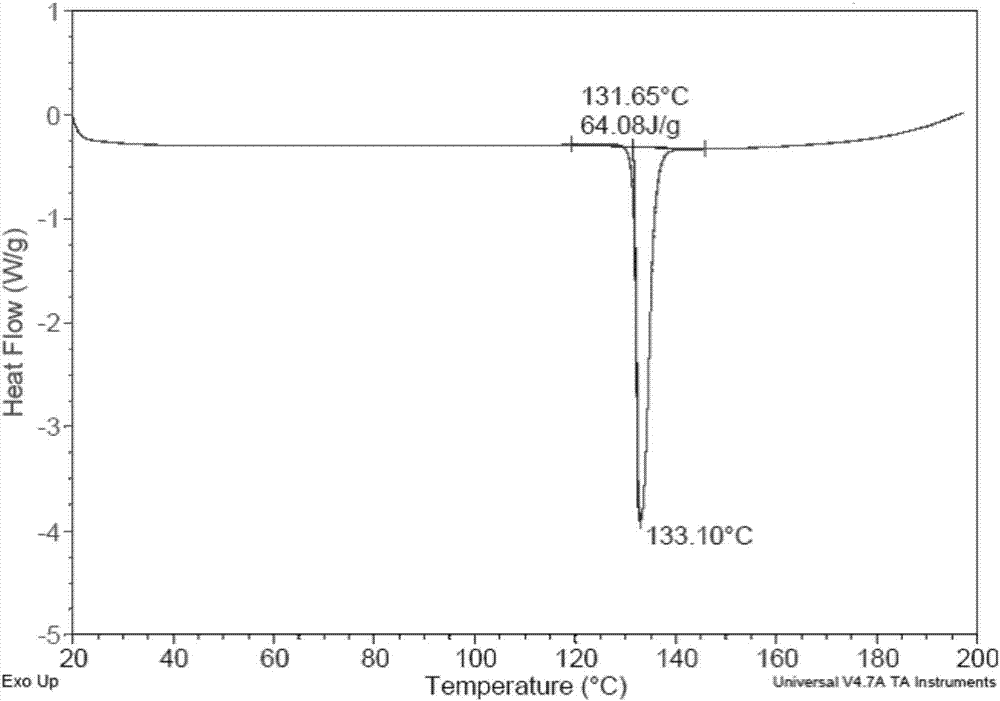
[0059] The XRD patterns measured using Cu-Kα radiation are as Figure 5 As shown, the spectral data are listed in Table 2 below. TGA test shows that the weight ...
Embodiment 2
[0062] Example 2: Preparation of Form II of tenofovir alafenamide fumarate
[0063] Take 500 mg of tenofovir alafenamide fumarate, add 5 mL of chloroform at room temperature, stir magnetically at room temperature for 3 days, centrifuge, and dry in vacuo overnight.
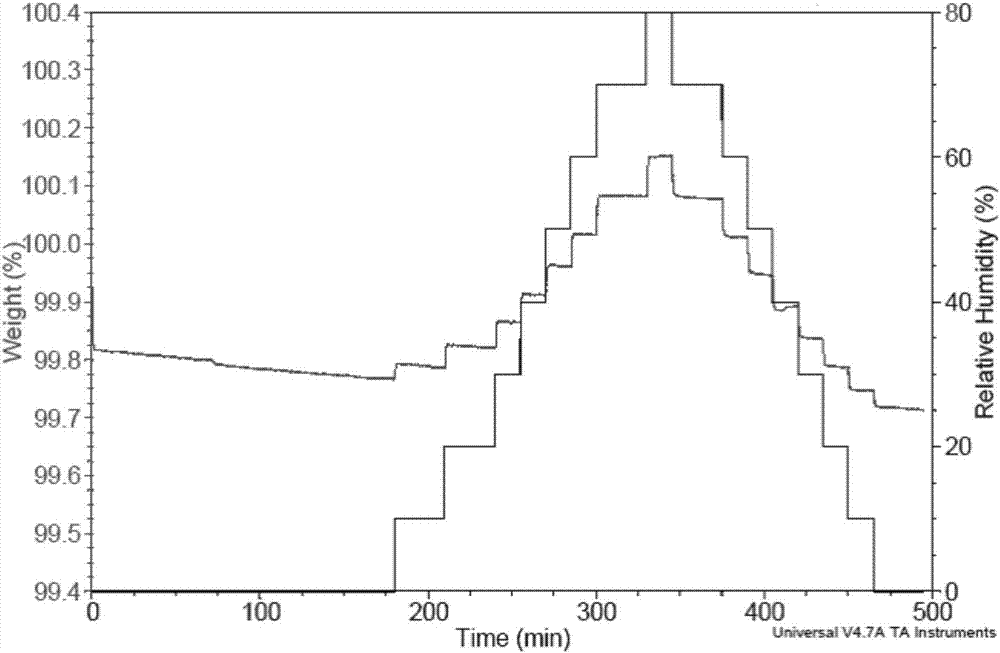
[0064] The XRD patterns measured using Cu-Kα radiation are as Figure 9 As shown, the spectral data are listed in Table 3 below. TAG detection shows that the weight loss is 3.2% before 100°C, which is about one water molecule (theoretical weight ratio is 3.0%), and the decomposition temperature is about 174°C. The crystal form is a monohydrate ( Figure 10 ). DSC detection shows that the sample has a broad absorption peak between 40°C and 85°C, and a crystal transformation exothermic peak between 90°C and 105°C, and the crystal transformation ( Figure 11 ).
[0065] Table 3. XRD pattern data of the crystal form II of tenofovir alafenamide fumarate
[0066] serial number
Embodiment 3
[0067] Example 3: Preparation of Form III of tenofovir alafenamide fumarate
[0068] Take 500 mg of tenofovir alafenamide fumarate, diffuse it in a trifluoroethanol solvent atmosphere at room temperature for 1 day, take it out and dry it at room temperature for 2 minutes.
[0069] The XRD patterns measured using Cu-Kα radiation are as Figure 12 As shown, the spectral data are listed in Table 4 below. The TGA spectrum shows that there is about 14.0% weight loss before 120°C, which is about one trifluoroethanol molecule, and the decomposition temperature is about 171°C ( Figure 13 ). The DSC spectrum shows that there is a desolvation endothermic peak between 50 and 105 ° C, and the melting point is about 120 ° C ( Figure 14 ).
[0070] Table 4. XRD pattern data of the crystal form III of tenofovir alafenamide fumarate
[0071]
[0072]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com