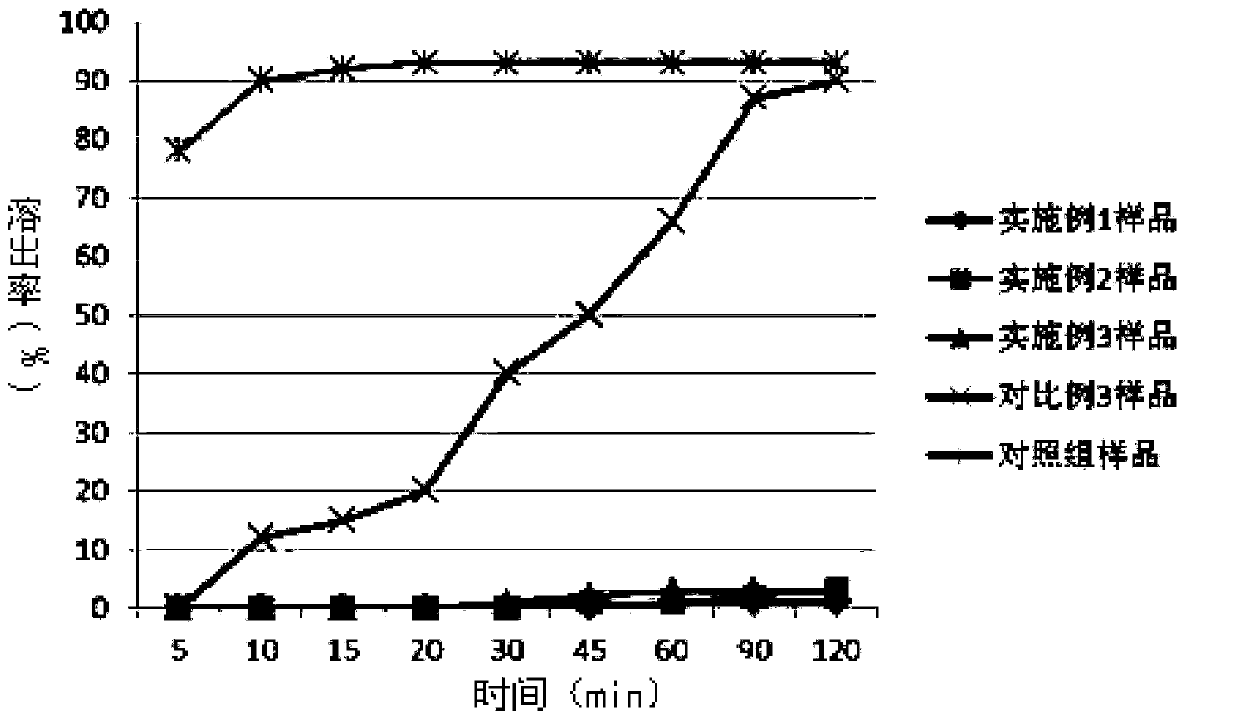
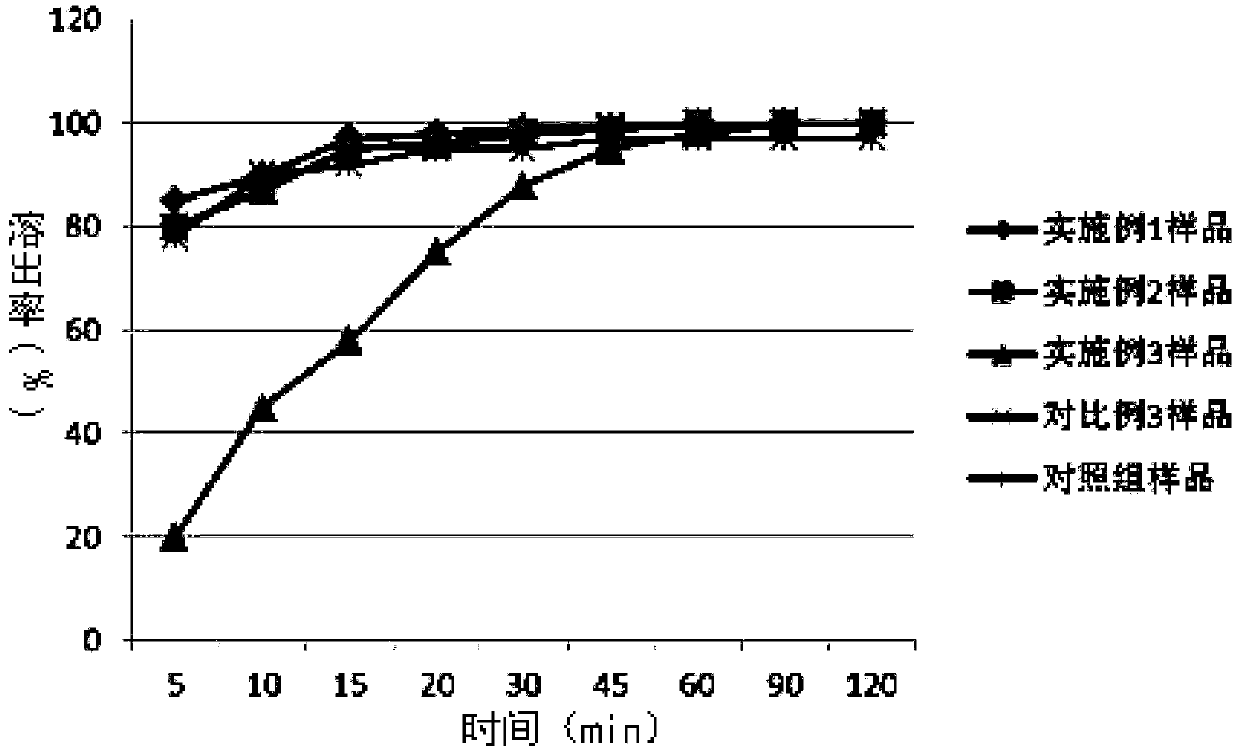
Tenofovir alafenamide enteric-coated tablet and preparation method thereof
A technology of alafenamide enteric and tenofovir, which is applied in the field of medicine, can solve problems such as the lack of research on alafenamide enteric-coated preparations, and achieve improved safety and effectiveness, complete coating, The effect of uniform intestinal distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
[0066] The preparation method comprises the steps of:
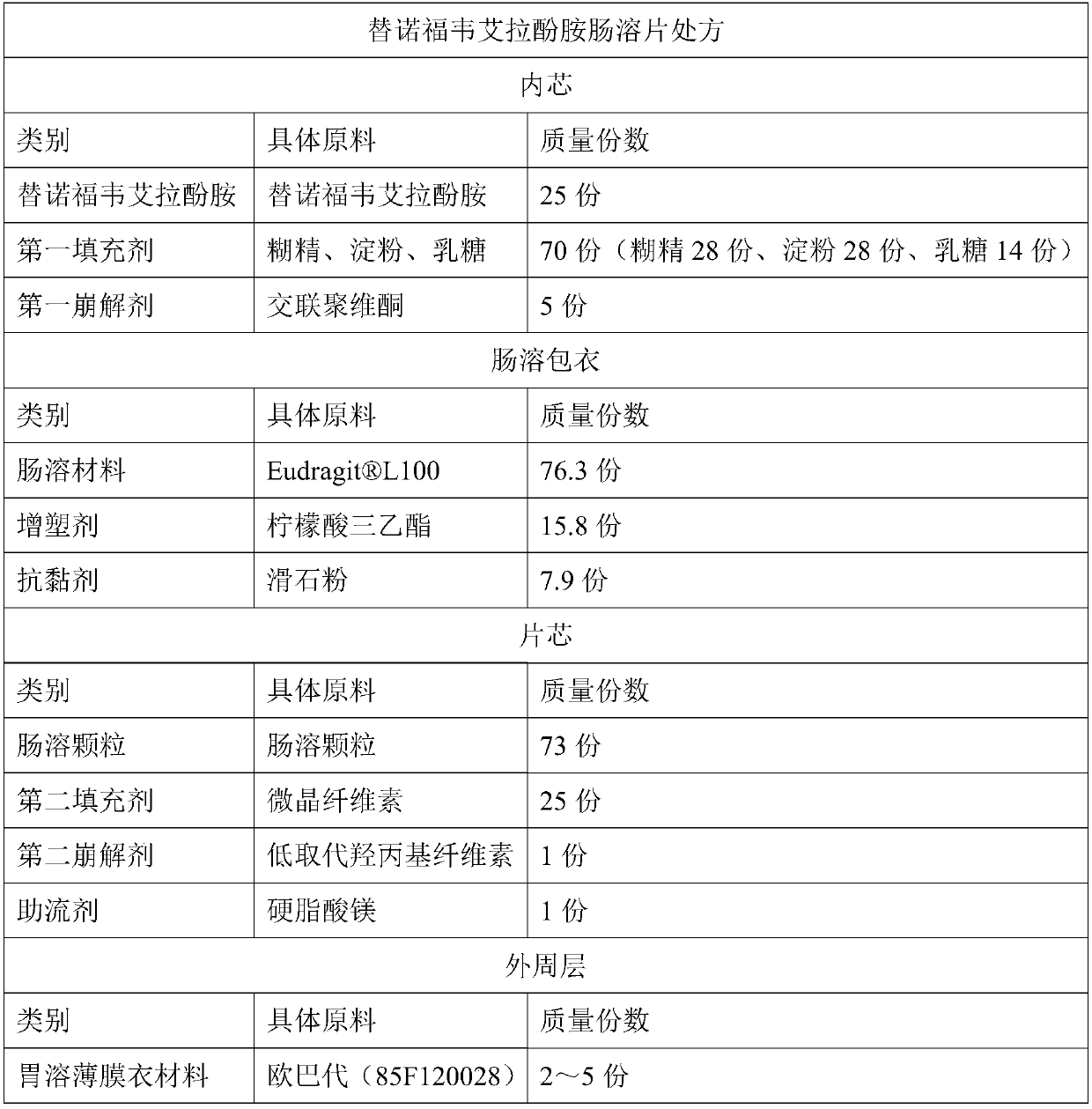
[0067] 1) Granulation: Weigh 25 parts of tenofovir alafenamide, 28 parts of dextrin, 28 parts of starch, 14 parts of lactose, and 5 parts of cross-linked povidone, and add them into a wet mixing granulator. Mixing, making soft materials, granulating, drying, granulating and other process steps to obtain granular inner cores;
[0068] 2) enteric coating: use 95% ethanol as solvent, add 76.3 parts of L100, stir until dissolved, continue to add 15.8 parts of triethyl citrate, 7.9 parts of talcum powder, stir and mix with a mixer for 45 minutes, then pass the suspension through an 80-mesh sieve to make an enteric coating solution, and use a flow Chemical bed coating technology, evenly spray the enteric coating solution on the granular inner core, until the weight of the enteric coating increases by 5.0-8.0%, and dry at 40-50°C for 3 hours to obtain enteric-coated granules;
[0069] 3) Tablet compression: Mix 73...
Embodiment 2
[0072]
[0073] The preparation method of the tenofovir alafenamide enteric-coated tablets in Example 2 is the same as in Example 1, except that the prescription of the tenofovir alafenamide enteric-coated tablets is the prescription in Example 2.
Embodiment 3
[0075]
[0076]
[0077] The preparation method of the tenofovir alafenamide enteric-coated tablets in Example 3 is the same as in Example 1, except that the prescription of the tenofovir alafenamide enteric-coated tablets is the prescription in Example 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com