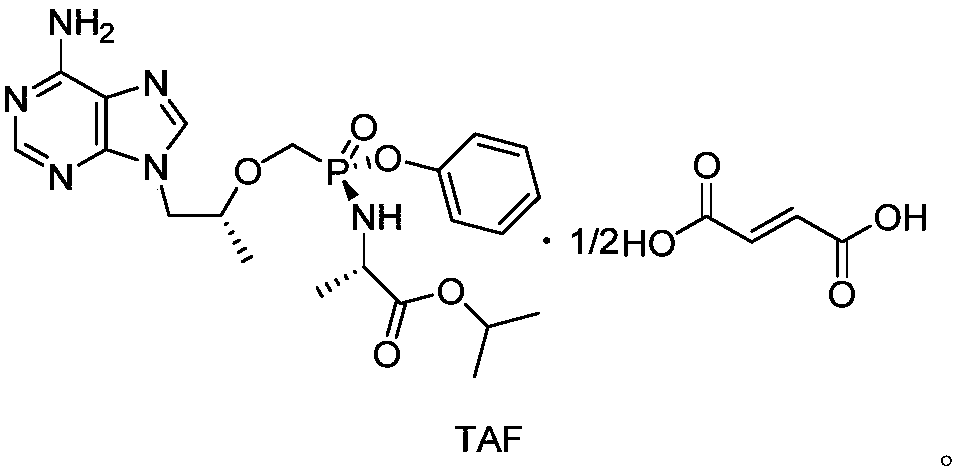
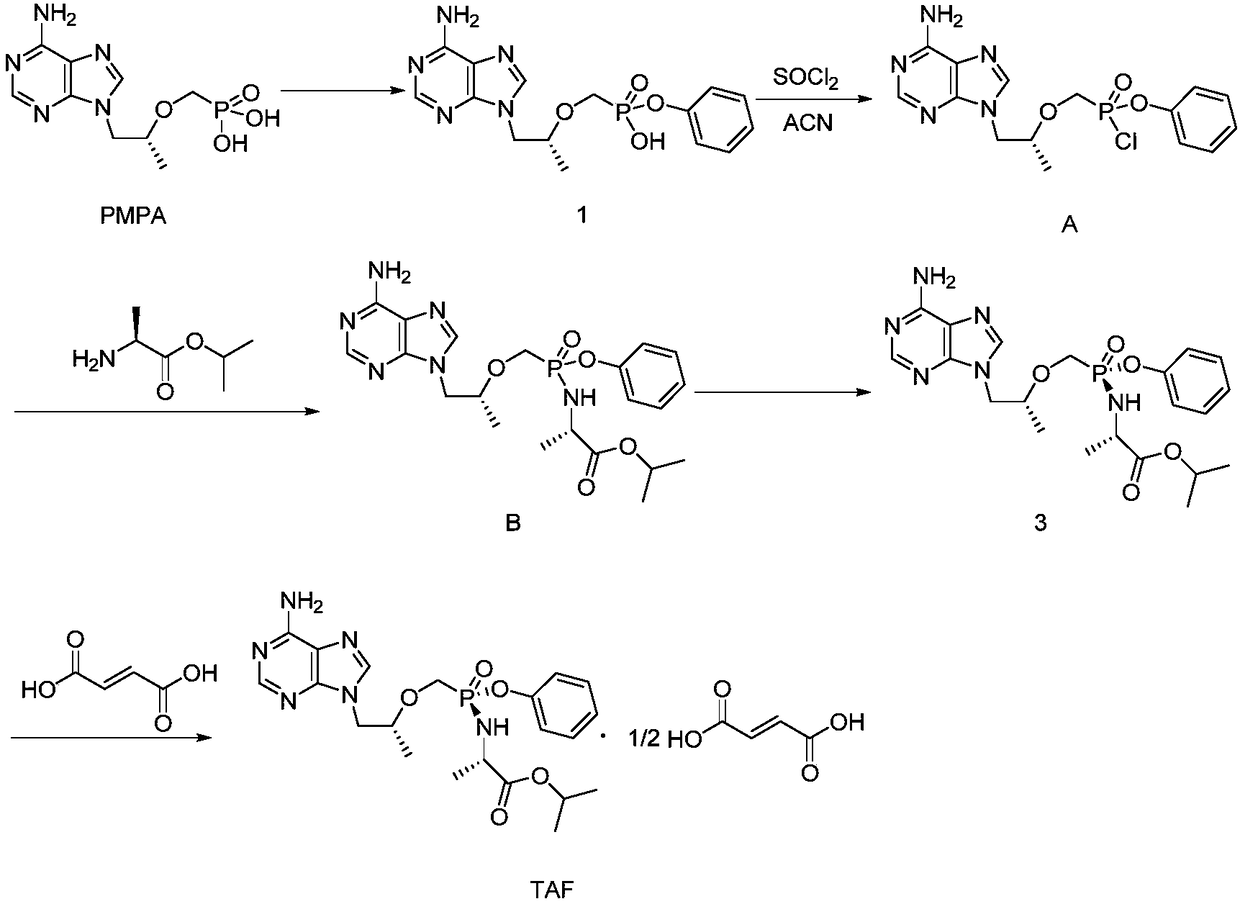
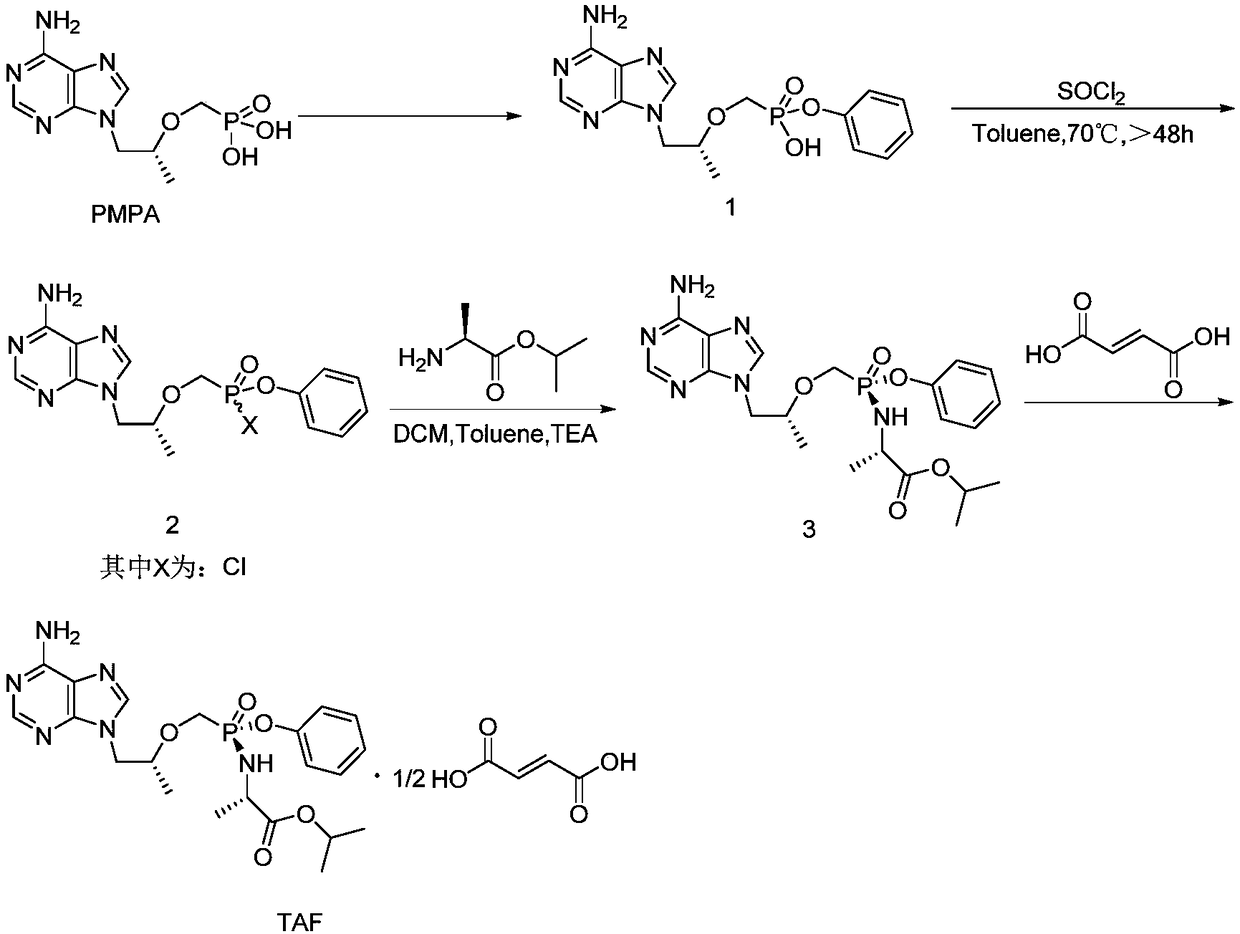
Preparation method of key intermediate TAF (tenofovir alafenamide fumarate)
A technology of tenofovir fumarate and alafenamide, which is applied in the field of drug synthesis, can solve the problems of increasing environmental protection pressure, not producing superior effects, increasing waste liquid discharge and treatment, etc., and achieving stable batch scale-up production and low cost Low, less by-product effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 high diastereoisomeric purity intermediate 2
[0045]
[0046] (1) Preparation of intermediate 1‐1:
[0047] Preparation of 9‐[(R)‐2[[(S)‐[di‐(phenoxy)phosphinyl]methoxy]propyl]adenine
[0048]At room temperature, add 25L of acetonitrile to a 100L glass-lined reactor, start stirring, then add tenofovir (5kg, 17.4mol), thionyl chloride (6.2kg, 52.1mol), and replace nitrogen protection (nitrogen replacement 3 Once, the vacuum degree of the reactor is ≤-0.080MPa, stir for 3-5 minutes, backfill nitrogen to normal pressure and stir for 3-5 minutes for a nitrogen replacement). After the addition is complete, raise the temperature to 70°C, keep the temperature for 2 hours, concentrate under reduced pressure, and then add 25L of acetonitrile and phenol (6.6kg, 69.6mol) into the glass-lined reactor, start stirring, and heat up to about 80°C. React for 13 hours, concentrate under reduced pressure, add 25L of ethyl acetate to the 100L glass-lin...
Embodiment 2
[0067] The nuclear magnetic data of embodiment 2-5 is consistent with embodiment 1 result.
Embodiment 6
[0068] The preparation of embodiment 6 high diastereoisomeric purity intermediate 2
[0069]
[0070] Add intermediate 1 9‐[(R)‐2‐[[(hydroxyphenoxyphosphono)methoxy]propyl]]adenine (10.0g, 27.5mmol) and 200.0g xylene in a 250ml three-necked flask , acetonitrile 20.0g, oxalyl chloride (34.9g, 275.0mmol), magnetic stirring, nitrogen replacement protection, heating up to 60°C, heat preservation reaction for 30h, cooling down to room temperature, concentrating under reduced pressure to remove oxalyl chloride, filtering, and vacuum drying to obtain off-white The solid is 9.9g, that is, intermediate 2, and the yield is 94%. Diastereomeric purity 91.59%. The NMR data is consistent with the result of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com