Tenofovir alafenamide compound, preparation method and purpose thereof
A technology of tenofovir alafenamide and compound, which is applied in the field of organic chemistry and can solve the problems of unpublished preparation method, cumbersome preparation process of hemi-fumarate, difficult to obtain and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
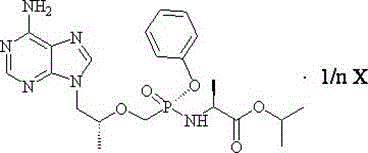
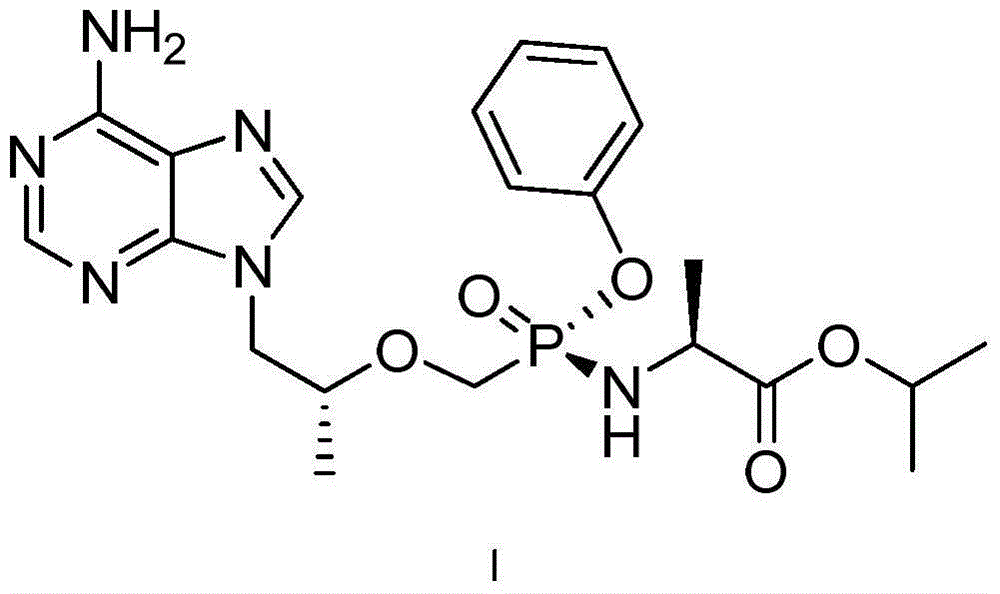
[0196] Preparation of tenofovir alafenamide (I)
[0197] At 20-25°C, 500.0 g (1.38 mol, 1.0 eq) of tenofovir monophenyl (prepared according to the method disclosed in CN1443189A) was added to 3.0 L of toluene, and the solid was completely dissolved under stirring, and then two Thionyl chloride was 150ml (2.05mol, 1.5eq), and the resulting mixture was heated to about 70°C and stirred for 96 hours. Concentrate to dryness under reduced pressure at 40-45°C, add 2.5L of toluene to the concentrate, add L-alanine isopropyl ester (commercially available) 813.5g (6.21mol, 4.5eq) dropwise at -10°C-10°C ) dissolved in 4.0L of dichloromethane, then stirred at -10°C-10°C for 30 minutes and then raised to room temperature, washed with 10% aqueous sodium dihydrogen phosphate solution 2.5L×2, separated the organic phase, and washed with 15% Potassium bicarbonate aqueous solution was washed with 1.0 L × 2, and then washed with 2.5 L of purified water. The obtained organic phase was dried over...
Embodiment 2
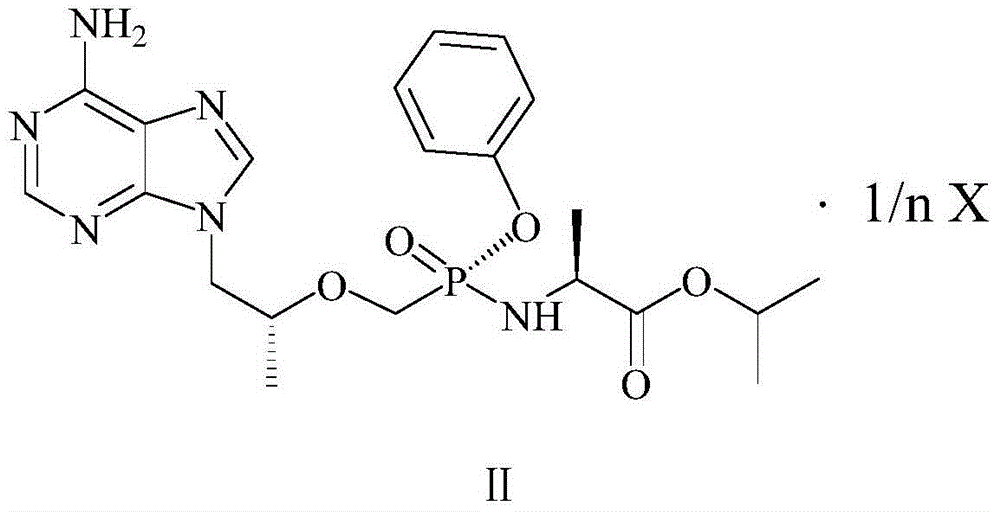
[0200] Preparation of L-tenofovir alafenamide tartrate (1:2)
[0201] At 70-75°C, dissolve 4.76g (10.0mmol) of tenofovir alafenamide and 0.75g (5.0mmol) of L-tartaric acid in 100ml of acetonitrile, stir and cool down to 15-20°C after complete dissolution, and then Continue to stir and crystallize; filter with suction, wash the filter cake with an appropriate amount of acetonitrile, and dry under reduced pressure at 40-45°C to obtain tenofovir alafenamide tartrate (1:2).
[0202] 1 HNMR (400MHz, DMSO-d 6 )δ: 8.14(s, 1H), 8.10(s, 1H), 7.31-7.27(t, 2H), 7.19(s, 2H), 7.15-7.11(m, 1H), 7.07-7.04(m, 2H) , 5.64-5.58(m, 1H), 4.90-4.80(m, 1H), 4.30-4.25(m, 2H), 4.18-4.12(m, 1H), 3.98-3.91(m, 1H), 3.89-3.81( m, 2H), 3.80-3.74 (m, 1H), 1.16-1.13 (t, 9H), 1.08-1.06 (d, 3H).
[0203] Melting range: 158-161°C.
[0204] the above 1In the HNMR results, the signal peaks with chemical shifts at δ8.14 (s, 1H) and 8.10 (s, 1H) were assigned to the two Hs on the adenine ring of tenofovir ala...
Embodiment 3
[0206] Preparation of Tenofovir Alafenamide D-Tartrate (1:1)
[0207] At 70-75°C, dissolve 4.76g (10.0mmol) of tenofovir alafenamide and 1.50g (10.0mmol) of D-tartaric acid in 100ml of acetonitrile, stir and cool down to 15-20°C after dissolution, continue Stir and crystallize; filter with suction, wash the filter cake with an appropriate amount of acetonitrile, and dry under reduced pressure at 40-45°C to obtain D-tenofovir alafenamide tartrate (1:1).
[0208] 1 HNMR (400MHz, DMSO-d 6 )δ: 8.15(s, 1H), 8.11(s, 1H), 7.31-7.28(t, 2H), 7.22(s, 2H), 7.15-7.12(m, 1H), 7.07-7.05(m, 2H) , 5.63-5.58(m, 1H), 4.90-4.81(m, 1H), 4.32-4.26(m, 3H), 4.18-4.13(m, 1H), 4.00-3.92(m, 1H), 3.90-3.81( m, 2H), 3.80-3.74 (m, 1H), 1.16-1.13 (t, 9H), 1.09-1.07 (d, 3H).
[0209] Melting range: 135-138°C.
[0210] the above 1 In the HNMR results, the signal peaks with chemical shifts at δ8.15 (s, 1H) and 8.11 (s, 1H) were assigned to the two Hs on tenofovir alafenamide adenine, δ4.32-4.26 (m, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com