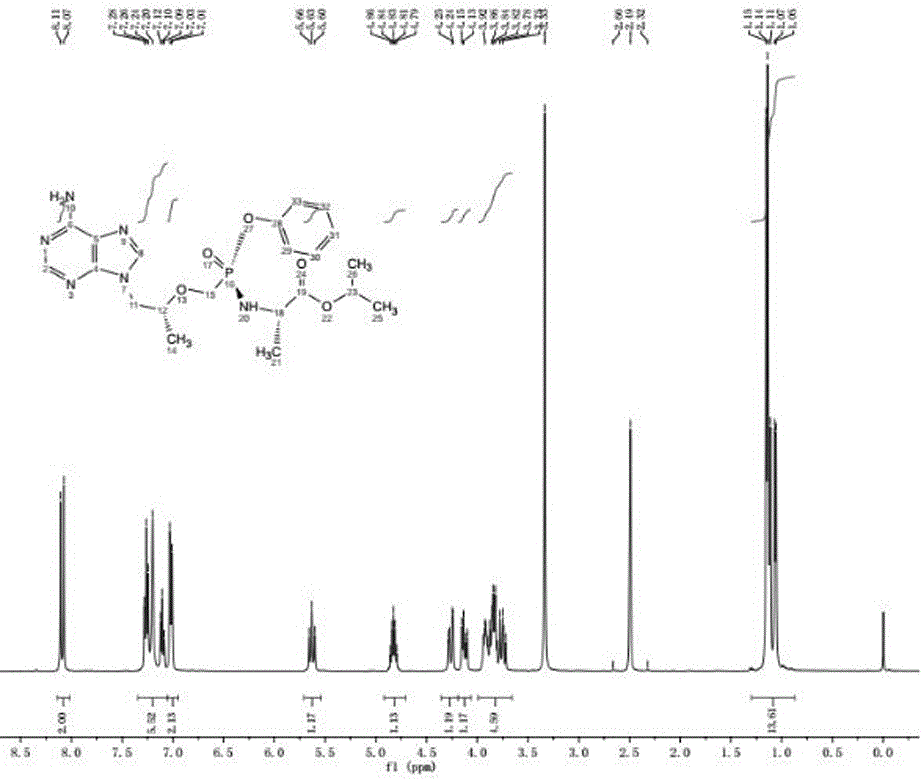
Preparation method of tenofovir alafenamide
A technology of tenofovir alafenamide and phenol, which is applied in the field of preparation of tenofovir alafenamide, can solve the problem of no large-scale supply of starting materials, achieve low cost, avoid operating procedures, The effect of simplifying the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] see figure 1 , a kind of preparation method of tenofovir alafenamide, concrete steps are as follows:
[0039] (1) PMPA is heated and reacted with chlorination reagents to obtain PMPA-2Cl; the chemical reaction formula is as follows:
[0040] (1);
[0041] (2) PMPA-2Cl was reacted with phenol and L-alanine isopropyl ester successively to obtain TAF-RS through a one-pot method; the chemical reaction formula is as follows:
[0042] (2);
[0043] (3) TAF-RS is purified to obtain tenofovir alafenamide; the chemical reaction formula is as follows:
[0044] (3).
[0045] The step (1) is reacted in an organic solvent or a solvent-free system; the organic solvent is acetonitrile, toluene or benzene.
[0046] The chlorination reagent can be selected from one of thionyl chloride, phosphorus trichloride, non-chlorinated phosphorus, and oxalyl chloride; further, preferably thionyl chloride;
[0047] The one-pot method in the step (2) is to react PMPA-2Cl with phenol in t...
Embodiment 1
[0052] A kind of preparation method of tenofovir alafenamide, concrete steps are as follows:
[0053] (1) PMPA is heated and reacted with chlorination reagents to obtain PMPA-2Cl; the chemical reaction formula is as follows:
[0054] (1);
[0055] Add PMPA (5g, 17.8mmol) to 25ml of acetonitrile, add thionyl chloride 10ml at room temperature, stir and heat up to reflux reaction for 2 hours, the solid in the system dissolves, sample is added to anhydrous methanol to monitor the complete conversion of raw materials ; The reaction solution is concentrated under reduced pressure, and the removal of solvent to obtain the foamy solid PMPA-2Cl weighs 6.5g;
[0056] (2) PMPA-2Cl was reacted with phenol and L-alanine isopropyl ester successively to obtain TAF-RS through a one-pot method; the chemical reaction formula is as follows:
[0057] (2);
[0058] Add 60ml of dichloromethane, 5g of PMPA-2Cl and 5.6g (55.2mmol) of triethylamine into a clean 250ml three-necked flask. After t...
Embodiment 2
[0063] A kind of preparation method of tenofovir alafenamide, concrete steps are as follows:
[0064] (1) PMPA is heated and reacted with chlorination reagents to obtain PMPA-2Cl; the chemical reaction formula is as follows:
[0065] (1);
[0066] PMPA (5g, 17.8mmol) was added to 20ml of thionyl chloride, stirred and heated to reflux for 1.5 hours. After the reaction, the solid in the system was dissolved, and samples were added to anhydrous methanol to monitor the complete conversion of raw materials. The reaction solution was concentrated to dryness under reduced pressure to obtain a foamy solid PMPA-2Cl, weighing 6.7 g;
[0067] (2) PMPA-2Cl was reacted with phenol and L-alanine isopropyl ester successively to obtain TAF-RS through a one-pot method; the chemical reaction formula is as follows:
[0068] (2);
[0069] Add 60ml of dichloromethane, 5g of PMPA-2Cl and 5.6g (55.2mmol) of triethylamine into a clean 250ml three-necked flask. After the addition, stir and cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com