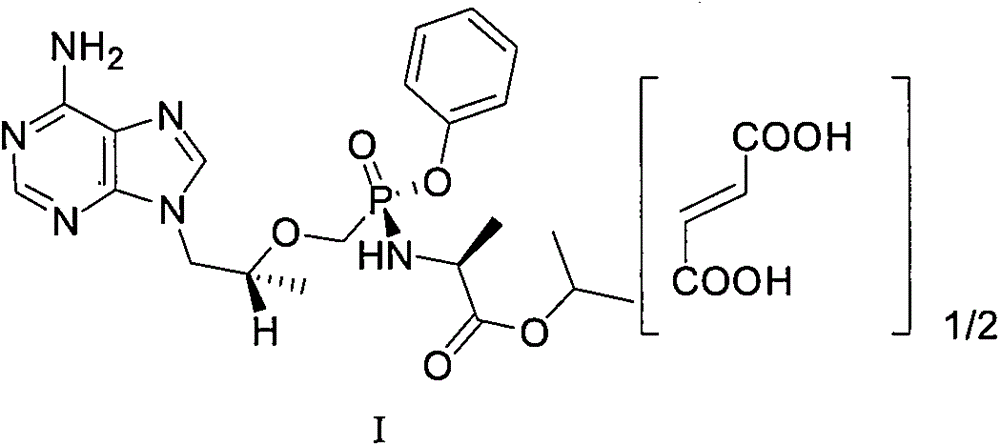
Preparation method and application for high-purity tenofovir alafenamide fumarate intermediate
A technology of tenofovir and tenofovir alafenamide, which is applied in the preparation and application of high-purity tenofovir fosprovir intermediates, can solve complex operations, high production costs, and cumbersome post-treatment processes and other problems, to achieve the effect of simple and safe operation, high product purity and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
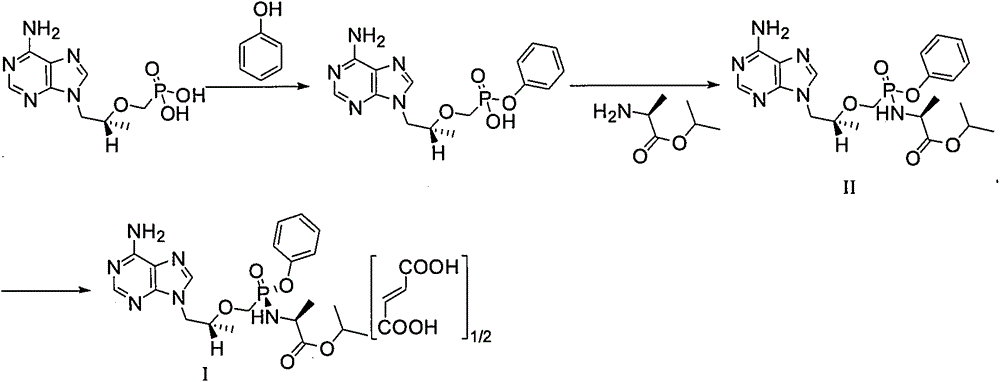
[0041] Example 1: The preparation method of tenofovir alafenamide intermediate III (reference US7803788 Example 2)
[0042]
[0043] Add 3.0Kg of tenofovir and 1.98Kg of phenol into 9L of N-methylpyrrolidone, heat to 60℃~70℃, add 1.68Kg of N,N-diisopropylethylamine and 3.53Kg of dicyclohexylcarbodiimide , heated to 90°C-100°C and stirred for 16 hours, cooled to 10-20°C, added dropwise 7.5L of water and stirred for 2 hours, filtered, and washed with 3L of water. The filtrate is adjusted to pH 11~12 with 20% sodium hydroxide aqueous solution (the described mass percentage refers to the percentage of the quality of sodium hydroxide in the total mass of sodium hydroxide aqueous solution) with a mass percentage of 20%, and is extracted with 7.5 L of ethyl acetate Twice, after separating the water phase, adjust the pH to 2.5-3.5 with concentrated hydrochloric acid, cool to 10-20°C and stir for 2 hours, filter, and dry in vacuum (-0.08--1.0MPa) at 40-50°C for 8-12 hours 2.67Kg of...
Embodiment 2
[0044] Example 2: Preparation method of tenofovir alafenamide II crude product (reference US7803788 Example 2)
[0045]
[0046] Add 2.40Kg of tenofovir alafenamide intermediate III (HPLC purity 95.85%) into 12L of acetonitrile, add 1.20Kg of thionyl chloride, heat to 75°C to 85°C and stir for 2 hours, then distill under reduced pressure after cooling most solvents. Dissolve the residue in 9L of dichloromethane, stir and cool to -25~-15℃, then add 1.97Kg of L-alanine isopropyl ester and 8.4L of dichloromethane, then add 1.1Kg of triethylamine, Stir at -15°C for 3 hours, then rise to room temperature (10°C to 35°C), and use 10% sodium dihydrogen phosphate aqueous solution (the mass percentage refers to the mass percentage of sodium dihydrogen phosphate to sodium dihydrogen phosphate The percentage of total mass of aqueous solution) 10L washes 4 times, is 10% sodium chloride aqueous solution 9L washes 1 time with mass percentage (the described mass percentage refers to the q...
Embodiment 3
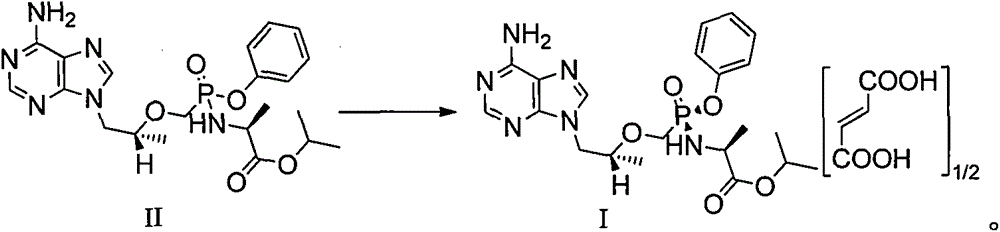
[0047] Embodiment 3: the preparation method of tenofovir alafenamide II
[0048] Tenofovir alafenamide II crude product 900g (HPLC purity 98.66%, chiral-HPLC purity 95.29%, wherein diastereoisomer 4.71%, enantiomer was not detected) was added acetonitrile 1.8L and In 7.2L of toluene, heat to 70℃~80℃ and stir at 120 rpm for 0.5 hours, slowly cool to 0~10℃ and stir at 60 rpm for 2 hours, filter, vacuum at 40~50℃ (-0.08~ -1.0 MPa) drying for 8-12 hours to obtain 760 g of tenofovir alafenamide II, with a yield of 84.4%. The HPLC purity is 99.87%, the largest single impurity is 0.04%, the chiral-HPLC purity is 99.98%, and the diastereoisomer is 0.02%, and the enantiomer is not detected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com