Tenofovir alafenamide hemifumarate compound, and pharmaceutical composition thereof
A technology for tenofovir alafenamide and fumarate compounds, which is applied in the fields of compounds of group 5/15 elements of the periodic table, organic chemistry, and pharmaceutical formulations, achieving excellent stability and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of Form C of tenofovir alafenamide hemifumarate
[0037] Take 500mg of tenofovir alafenamide hemifumarate, add 25mL of acetone and heat to 50°C to dissolve, and dry the solvent with nitrogen at room temperature to obtain the product.
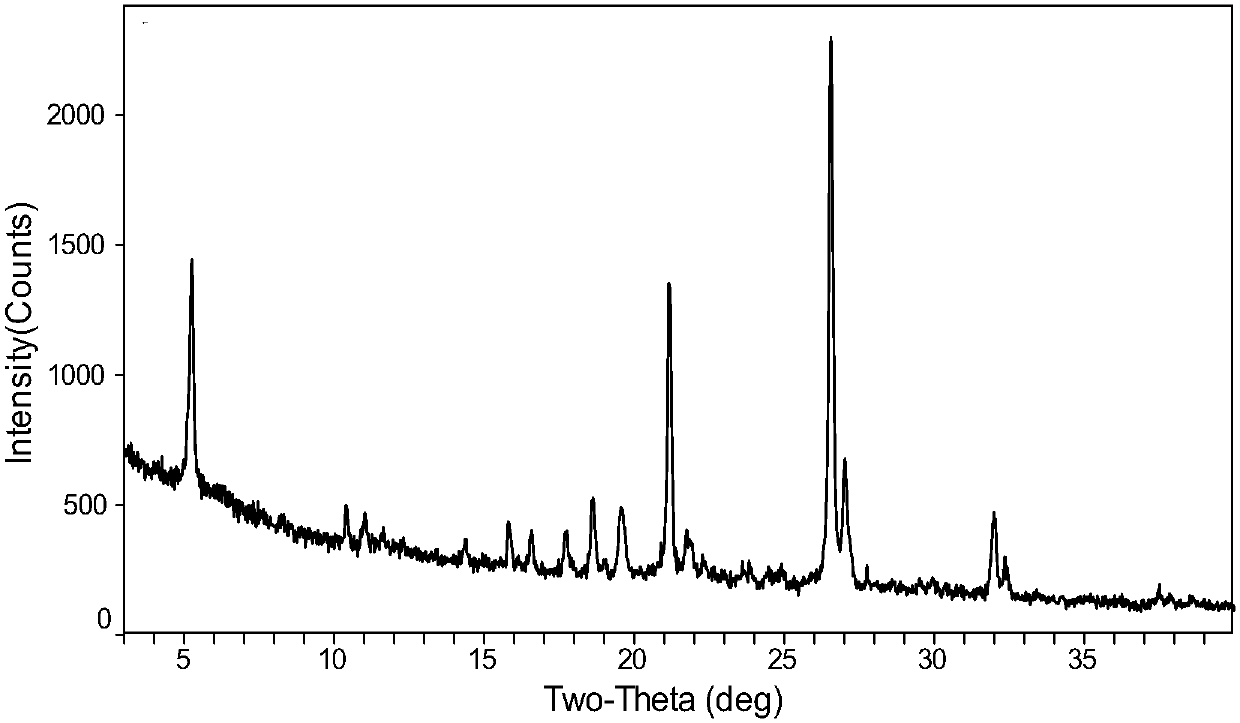
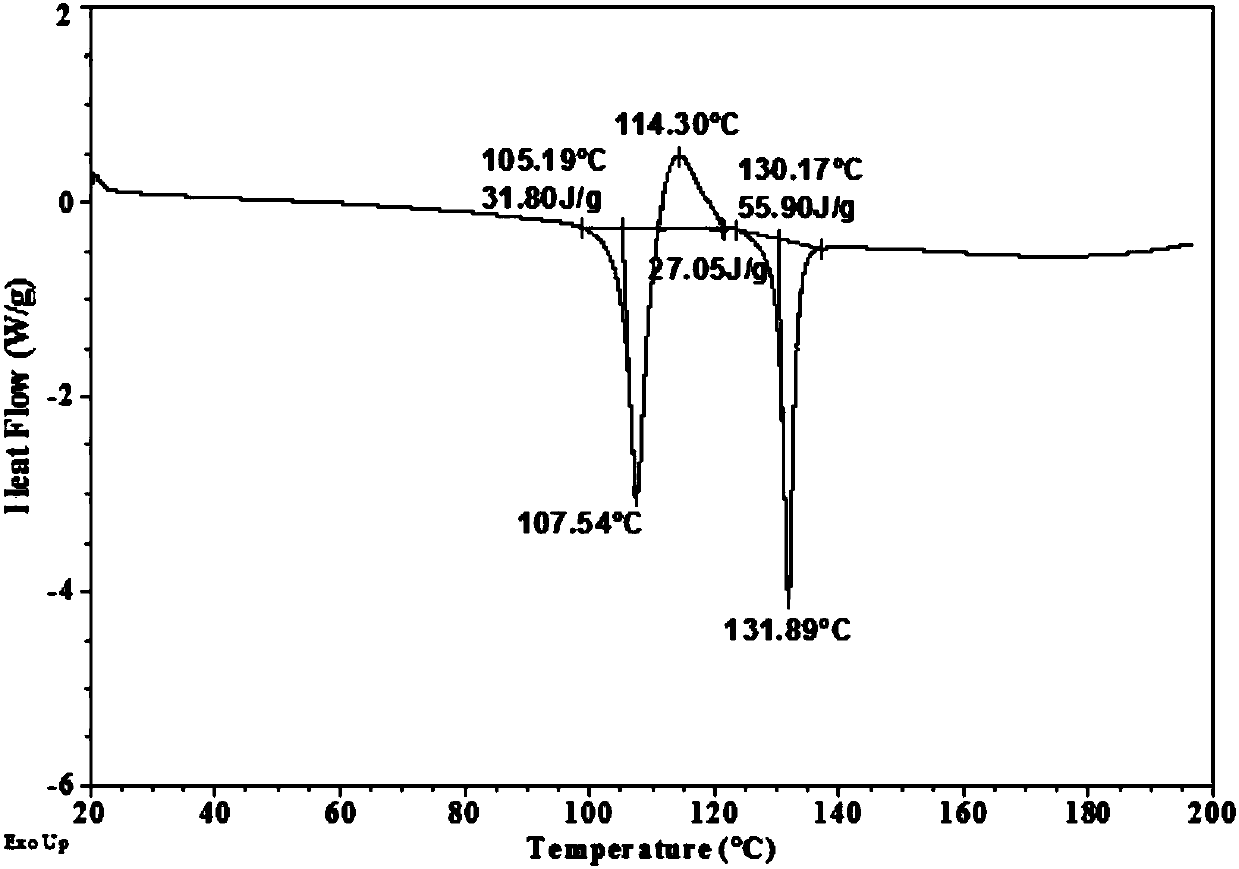
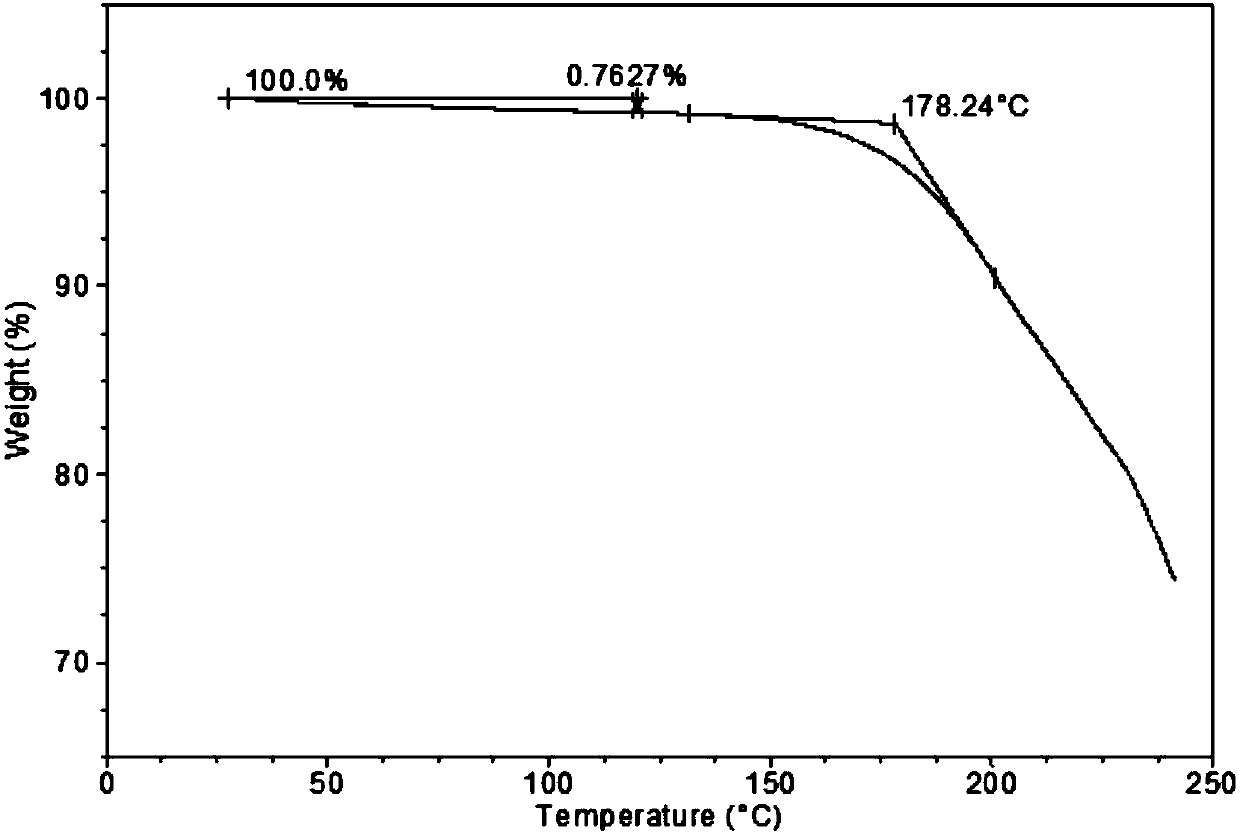
[0038] XRD patterns such as figure 1 shown. The DSC spectrum shows that the melting point of the sample is about 105°C, and the melting is accompanied by exothermic crystallization, and the melting point of the sample after crystallization is about 130°C ( figure 2 ). The TGA spectrum shows that the weight loss is 0.8% before 120°C, the sample is anhydrous, and the decomposition temperature is about 178°C ( image 3 ).
Embodiment 2
[0039] Example 2: Preparation of E crystal form of tenofovir alafenamide hemifumarate
[0040] Take 100 mg of tenofovir alafenamide hemifumarate and diffuse it in a trifluoroethanol solvent atmosphere at room temperature for 4 hours.
[0041] XRD patterns such as Figure 4 shown. The DSC spectrum shows that there is a small and broad endothermic peak at 60-90°C, and the melting point of the sample after desolvation is 130°C, and crystal transformation occurs ( Figure 5). TGA spectrum shows 7.1% weight loss before 120 ℃, close to half trifluoroethanol molecule (theoretical weight ratio is 8.6%), sample is half trifluoroethanol solvate, and decomposition temperature is about 181 ℃ ( Figure 6 ).
Embodiment 3
[0042] Example 3: Preparation of H crystal form of tenofovir alafenamide hemifumarate
[0043] Take 100mg of tenofovir alafenamide hemifumarate, add 1,4-dioxane at room temperature, heat to 45°C to dissolve, filter, cool at 4°C for crystallization, a small amount of sample is precipitated, nitrogen blowing Can do it.
[0044] XRD patterns such as Figure 7 shown. The DSC pattern shows that desolvation is accompanied by melting, and the onset temperature is about 121 ° C ( Figure 8 ). The TGA spectrum shows a weight loss of 6.9% before 120°C, which is close to half of a 1,4-dioxane molecule (theoretical weight ratio is 7.6%). The sample is a half 1,4-dioxane solvate, and the decomposition temperature is about 180℃( Figure 9 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com