Preparation method and application of process impurity of tenofovir alafenamide fumarate
A technology of fumarate and propionate, which is applied in the field of drug synthesis and can solve the problems of no process impurity reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: (S)-isopropyl 2-(((R,S)-((((R)-1-(6-amino-9H-9-purinyl)-2-propyl)oxy) Synthesis of methyl)(methoxy)phosphinyl)amino)propionate.
[0081] Add 50ml of methanol and 10ml of purified water to a 250ml three-necked flask, and add 5.92g of 9-[(R)-2-[[(R,S)-[[(S)-1-(isopropoxycarbonyl) under stirring Ethyl] amino] phenoxyphosphinyl] methoxy] propyl] adenine fumarate (TAF-3), under nitrogen protection, cool down to 10-20°C, add 1.20g of NaOH, complete addition, stir overnight. Add 100ml of dichloromethane, 50ml of purified water, separate the liquids, collect the organic phase, and distill under reduced pressure to obtain a solid crude product, which will be purified by column chromatography. The elution ratio is: methanol:dichloromethane=1:20, collect The eluent was 230ml in total, temperature controlled at 30-40°C, vacuum degree: -0.08MPa, distilled under reduced pressure, and the solvent was distilled until no distillate was distilled to obtain 0.67g of yellow oi...
Embodiment 2
[0082]Example 2: (S)-isopropyl 2-(((R,S)-((((R)-1-(6-amino-9H-9-purinyl)-2-propyl)oxy) Separation of methyl)(methoxy)phosphinyl)amino)propionate
[0083] 0.67g (S)-isopropyl 2-(((R,S)-((((R)-1-(6-amino-9H-9-purinyl)-2-propyl)oxy)methyl Base) (methoxy)phosphinyl)amino)propionate was added to 50ml of ethanol for dissolution, and the sample was separated by supercritical fluid chromatography (model: MD-2018Plus). Column model: DAICEL CHIRALCEL AS-H (0.46cm I.D.x 25cm L), column temperature 35°C, detection wavelength: 220nm, injection concentration: 20mg / ml, CO 2 / EtOH=70 / 30, flow rate 2.0ml / min, time 10 minutes. After separation, the collected liquids were combined, controlled at a temperature of 30-40°C, vacuum: -0.08MPa, distilled under reduced pressure, and distilled off the solvent until no distillate was distilled to obtain 300.3 mg of (S)-isopropyl 2-(((S) -((((R)-1-(6-amino-9H-9-purinyl)-2-propyl)oxy)methyl)(methoxy)phosphinyl)amino)propionate (compound B free base), o...
Embodiment 3
[0084] Example 3: (S)-isopropyl 2-(((R)-((((R)-1-(6-amino-9H-9-purinyl)-2-propyl)oxy)methyl ) Synthesis of (methoxy)phosphinyl)amino)propionate fumarate (compound A).
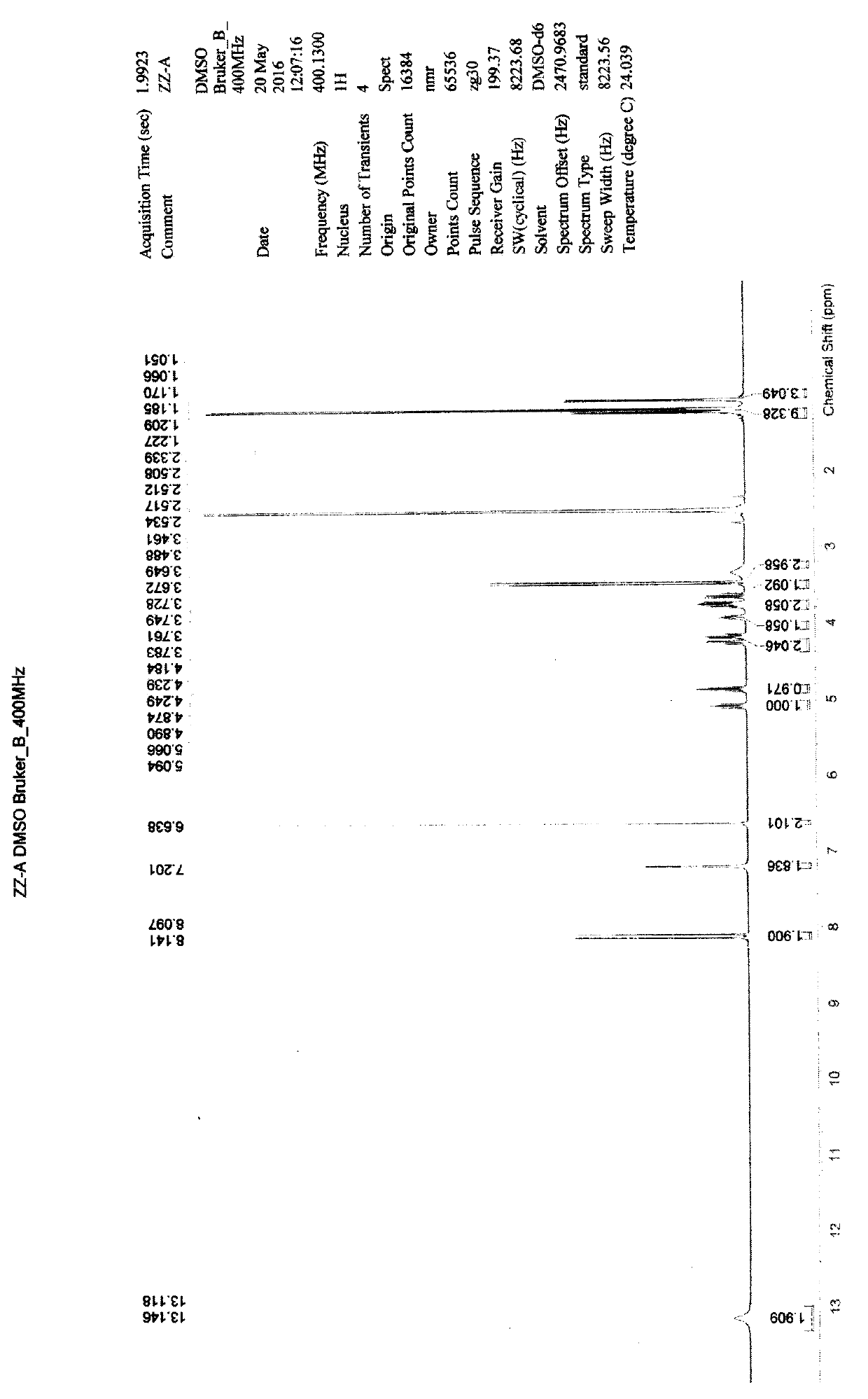
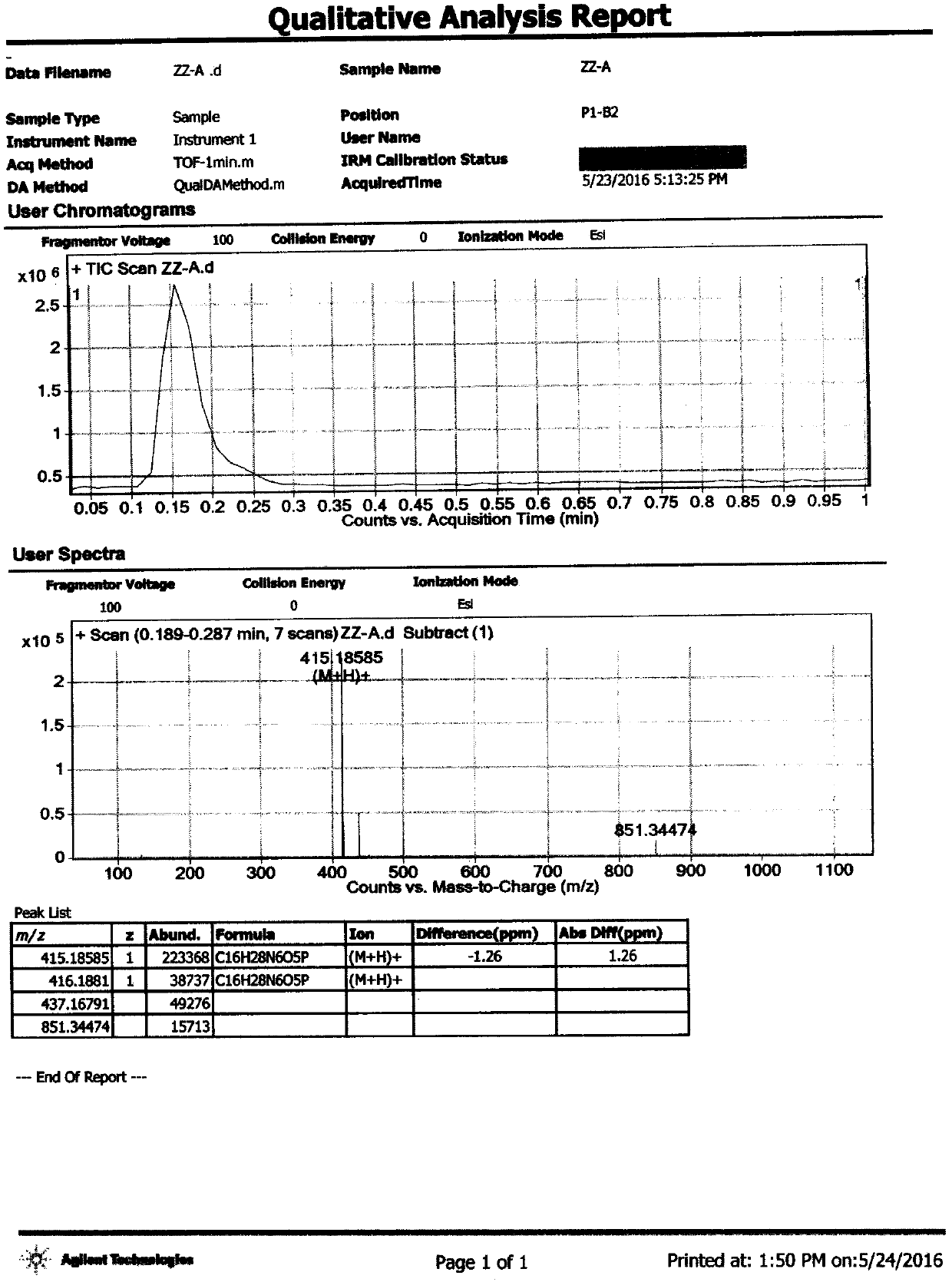
[0085] Add 10ml of acetonitrile to a 50ml three-necked flask, and add 300.3mg of (S)-isopropyl 2-(((R)-((((R)-1-(6-amino-9H-9-purine Base)-2-propyl)oxy)methyl)(methoxy)phosphinyl)amino)propionate, 84.1mg fumaric acid, under nitrogen protection, heated to reflux, and kept stirring for 2 hours. Control the temperature at 30-40°C, vacuum degree: -0.08MPa, distill under reduced pressure, and distill off the solvent until no distillate is distilled to obtain Compound A, 345.7 mg of white powder, yield: 89.94%. 1 H-NMR (400Mz, DMSO-d 6 )δ: 13.146 (br, s, 2H), 8.141 (s, 1H), 8.097 (s, 1H), 7.201 (br, s, 2H), 6.638 (s, 2H), 5.092 (dd, J=10.4Hz , J=21.6Hz, 1H), 4.890~4.874(m, 1H), 4.249~4.184(m, 2H), 3.949~3.908(m, 1H), 3.783~3.728(m, 2H), 3.672~3.645(m , 1H), 3.461(d, J=10.8Hz, 3H), 1.209(d, J=7.2Hz, 3H), 1.170(d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com