HPLC detecting method for tenofovir alafenamide and isomer thereof
A technology for tenofovir alafenamide and a detection method is applied in the field of HPLC detection of tenofovir alafenamide and its isomers, and can solve the problem of long running time and inability to tenofovir alafenamide. The separation of amine and its isomers impurities, the complex mobile phase preparation and other problems, to achieve the effect of accurate results and good resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A method for separating and determining tenofovir alafenamide and its isomers, specifically comprising the steps of:
[0043] 1) Column: Kromstar C 18 (4.6×250 mm×5 µm);
[0044] 2) Column temperature: 30°C;
[0045] 3) Wavelength: 260nm;
[0046] 4) Injection volume: 5 µL;
[0047] 5) Flow rate: 1.0 mL / min;
[0048] 6) Isocratic elution: methanol: 0.1% phosphoric acid aqueous solution = 40:60.
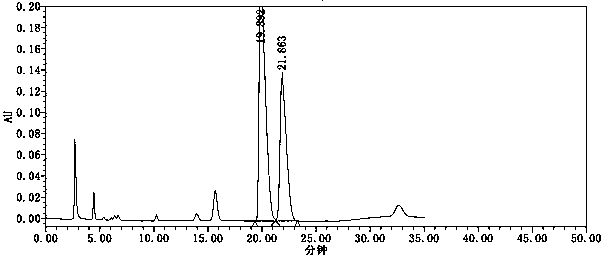
[0049] see results figure 1 , figure 1 The chromatographic peak at 19.892 min is the chromatographic peak of the isomers of tenofovir alafenamide, and the chromatographic peak at 21.863 min is the chromatographic peak of tenofovir alafenamide. The separation degree of the two was 1.83, meeting the requirements of the Chinese Pharmacopoeia.
Embodiment 2
[0051] A method for separating and determining tenofovir alafenamide and its isomers, specifically comprising the steps of:
[0052] 1) Column: Kromstar C 18 (4.6×250 mm×5 µm);
[0053] 2) Column temperature: 30°C;
[0054] 3) Wavelength: 260nm;
[0055] 4) Injection volume: 5 µL;
[0056] 5) Flow rate: 1.0 mL / min;
[0057] 6) Isocratic elution: methanol: 0.1% formic acid aqueous solution = 40:60.
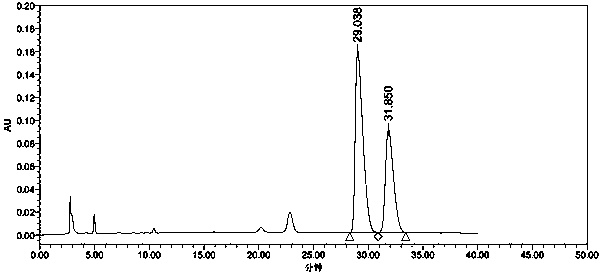
[0058] see results figure 2 , figure 2 The chromatographic peak at 29.038 min is the chromatographic peak of the isomers of tenofovir alafenamide, and the chromatographic peak at 31.850 min is the chromatographic peak of tenofovir alafenamide. The separation degree of the two is 2.13, meeting the requirements of the Chinese Pharmacopoeia.
Embodiment 3
[0060] A method for separating and determining tenofovir alafenamide and its isomers, specifically comprising the steps of:
[0061] 1) Column: Kromstar C 18 (4.6×250 mm×5 µm);
[0062] 2) Column temperature: 30°C;
[0063] 3) Wavelength: 260nm;
[0064] 4) Injection volume: 5 µL;
[0065] 5) Flow rate: 1.0 mL / min;
[0066] 6) Isocratic elution: Methanol: 0.1% trifluoroacetic acid aqueous solution = 40:60.
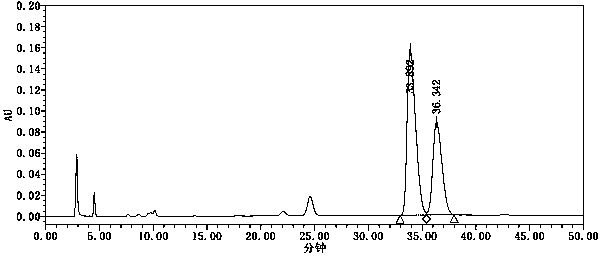
[0067] see results image 3 , image 3The chromatographic peak at 33.892 min is the chromatographic peak of the isomers of tenofovir alafenamide, and the chromatographic peak at 36.342 min is the chromatographic peak of tenofovir alafenamide. The separation degree of the two is 1.62, meeting the requirements of the Chinese Pharmacopoeia.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com