Preparation method of fumaric acid tenofovir alafenamide
A technology of tenofovir fumarate and alafenamide, which is applied in the field of medicine and can solve problems such as affecting product yield and difficulty in purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
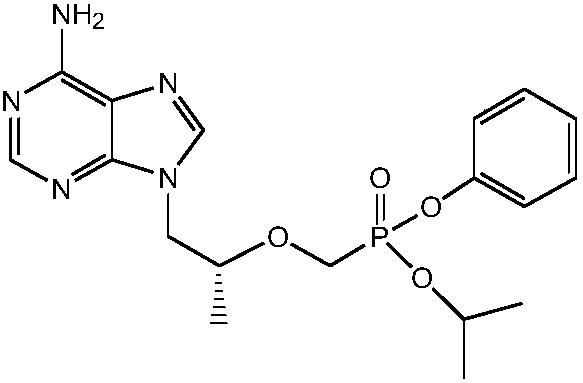
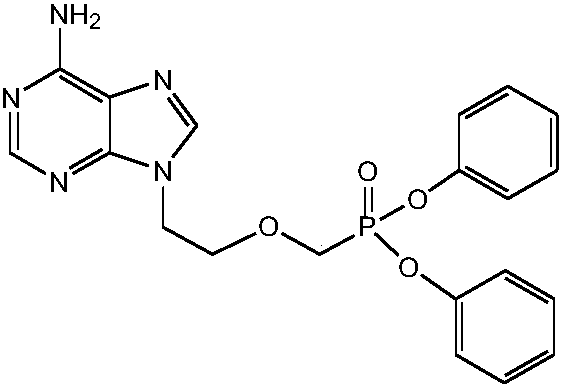
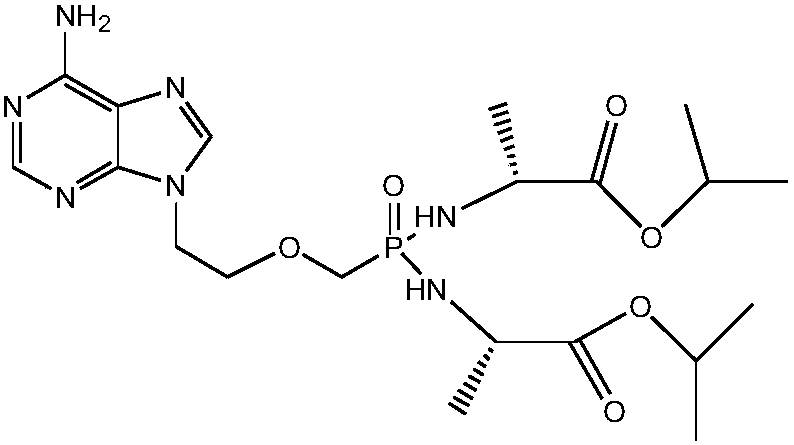
[0032] In order to solve the above-mentioned technical problems, the invention provides a kind of preparation method of tenofovir alafenamide fumarate, comprising the following steps:
[0033] (1) Esterification: add acid-binding agent, (R)-tenofovir, triphenyl phosphite, the first organic solvent into the reactor, reflux reaction for 60-80h, after the reaction is over, concentrate under reduced pressure to remove the first Organic solvent, cool the residue to room temperature, add water, ethyl acetate, stir to dissolve, separate layers, extract the water phase with ethyl acetate, adjust the pH value of the water phase to 2-3 with concentrated hydrochloric acid, cool down and crystallize, filter, and use Rinse with dilute hydrochloric acid with a mass fraction of 1%, then stir with dichloromethane at room temperature, filter, rinse with dichloromethane, and dry under atmospheric pressure to obtain TAF-1;
[0034] (2) Halogenation: under the protection of inert gas, add TAF-1 a...
Embodiment 1
[0070] The preparation method of tenofovir alafenamide fumarate comprises the following steps:
[0071] (1) Esterification: add acid-binding agent, (R)-tenofovir, triphenyl phosphite, the first organic solvent into the reactor, reflux reaction for 70h, after the reaction is over, concentrate under reduced pressure to remove the first organic solvent , the residue was cooled to room temperature, added water, ethyl acetate, stirred to dissolve, layered, the aqueous phase was extracted with ethyl acetate, and the aqueous phase was adjusted to a pH value of 2-3 with concentrated hydrochloric acid with a mass fraction of 36%, cooling and crystallization , filtered, rinsed with dilute hydrochloric acid with a mass fraction of 1%, then stirred at room temperature with dichloromethane (the (R)-tenofovir and the first organic solvent, the water, the ethyl acetate , the weight ratio of the dichloromethane is 1:9:3:8:7), filtered, washed with dichloromethane, and air-dried at normal pres...
Embodiment 2
[0077] The preparation method of tenofovir alafenamide fumarate comprises the following steps:
[0078] (1) Esterification: add acid-binding agent, (R)-tenofovir, triphenyl phosphite, the first organic solvent into the reactor, reflux reaction for 70h, after the reaction is over, concentrate under reduced pressure to remove the first organic solvent , the residue is cooled to room temperature, add water, ethyl acetate, stir to dissolve, separate layers, extract the water phase with ethyl acetate, adjust the pH value of the water phase to 2-3 with concentrated hydrochloric acid with a mass fraction of 36%, cool down and crystallize , filtered, rinsed with dilute hydrochloric acid with a mass fraction of 1%, then stirred at room temperature with dichloromethane (the (R)-tenofovir and the first organic solvent, the water, the ethyl acetate , the weight ratio of the dichloromethane is 1:9:3:8:7), filtered, washed with dichloromethane, and air-dried at normal pressure to obtain TAF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com