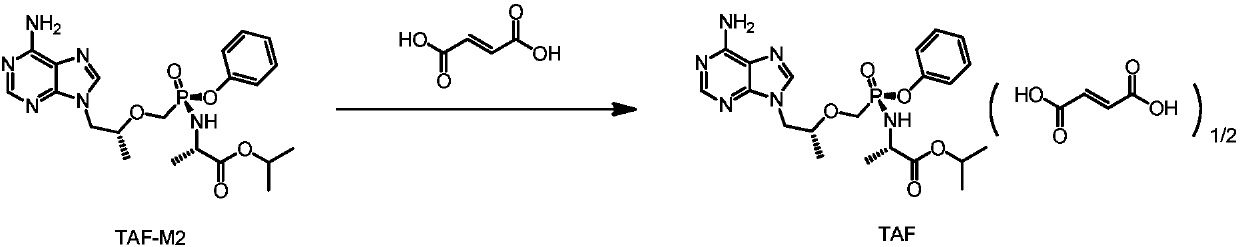
Preparation method of tenofovir alafenamide hemifumarate
A technology of tenofovir alafenamide and hemi-fumarate, which is applied in the preparation of carboxylate, the preparation of carboxylate, the preparation of organic compounds, etc. Problems such as poor operability, to avoid process operation and purification methods, to achieve the effect of accurate ratio and precise control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
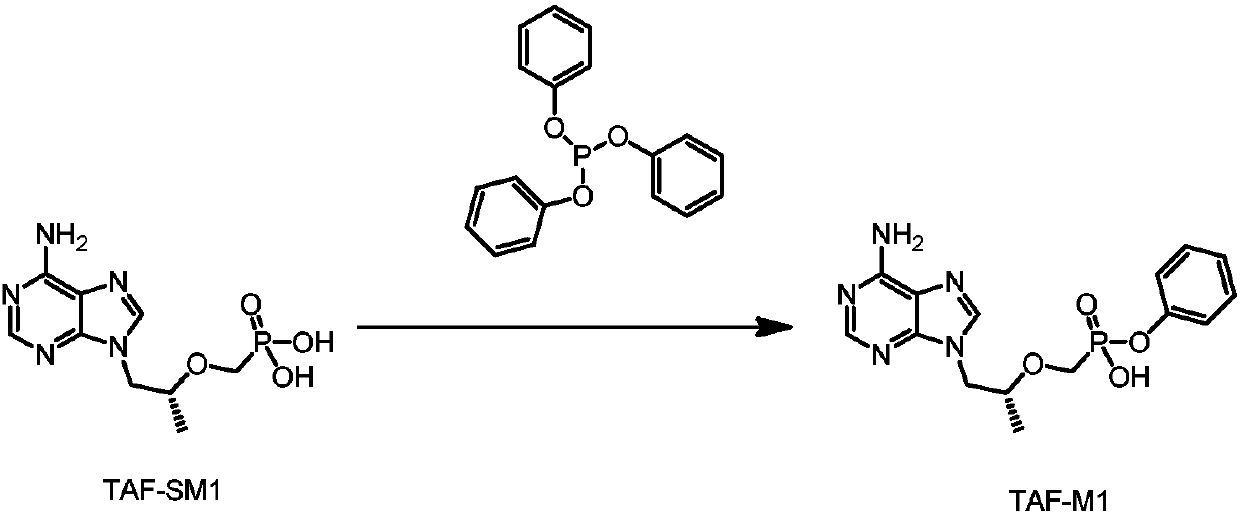
[0038] The preparation of formula TAF-M1 compound
[0039] Add 4L of acetonitrile to the 10L reaction flask, then add TAF-SM1 (500g), DMAP (213g), Et 3 N (352g), triphenyl phosphite (810g), heat up to 70-80°C for reflux reaction for 65-72 hours after addition; 30°C), add water (2000g) and EA (1500ml), stir to dissolve, separate layers, extract the water phase with 1500ml*3 EA three times, adjust the pH value of the water phase to 1-2 (about 160°C) with concentrated hydrochloric acid at 20-30°C -170ml), stirred for 30 minutes, cooled to 0-10 to crystallize for 1-2 hours after the solid was precipitated, filtered, rinsed with 1% dilute hydrochloric acid (0-10°C), filtered and dried, and the wet cake was beaten with 1.5L of DCM After 2-3 hours, it was filtered and air-dried at normal pressure for 24-30 hours, and 474 g of white solid was collected, namely the compound of formula TAF-M1, with a purity of more than 98% by HPLC.
Embodiment 2
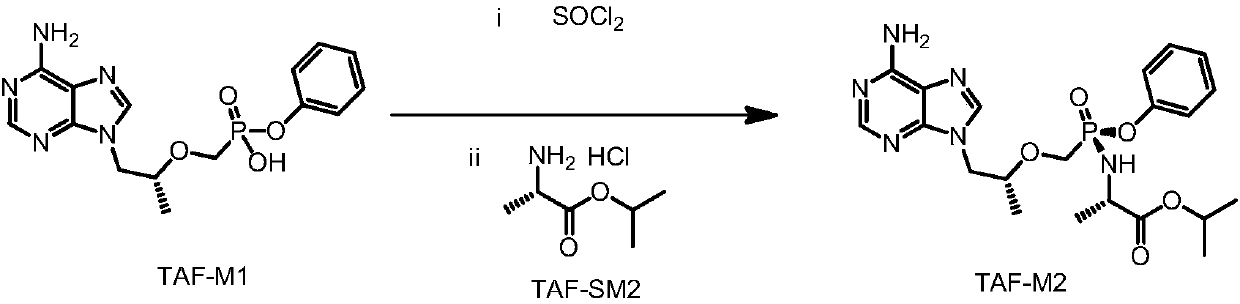
[0041] The preparation of formula TAF-M2 compound
[0042] Acyl chloride step: 2.4L of toluene was added to a 5L reaction flask, TAF-M1 (400g, prepared according to the method in Example 1) was added, heated to 70-80°C under nitrogen protection, and thionyl chloride (262g ), keep warm at 70-80°C and react for 40-50 hours, control the temperature below 75°C, concentrate under reduced pressure until no obvious fraction comes out, under the protection of nitrogen, cool down to 20-40°C, add 1600ml of dichloromethane, stir and disperse, stand-by;
[0043] Isolation step: Add 2.4L of dichloromethane into a 5L reaction flask, add TAF-SM2 (646g) and potassium bicarbonate (441g) to free at 15-25°C for 5-10 hours, filter, drain, and control the temperature for 30- Concentrate to dryness under reduced pressure at 40°C, and take it twice with 400ml*2 of new dichloromethane, and add 2.4L of new dichloromethane to the residue.
[0044] Reaction steps: Cool to -25~-15°C under nitrogen prot...
Embodiment 3
[0048] The preparation of formula TAF-M2 compound
[0049] Acyl chloride step: 2.4L of chlorobenzene was added to a 5L reaction flask, TAF-M1 (400g, prepared according to the method in Example 1) was added, the temperature was lowered to 70-80°C under nitrogen protection, and thionyl chloride was slowly added dropwise ( 262g), after adding, keep warm at 70-80°C and react for 50-60 hours, control the temperature below 75°C, concentrate under reduced pressure until no obvious fraction comes out, add 400ml of chlorobenzene, concentrate under reduced pressure until no obvious fraction comes out, under nitrogen protection, Cool down to 20-40°C, add 1600ml of dichloromethane, stir to disperse, and set aside;
[0050] Isolation step: Add 2.4L of dichloromethane into a 5L reaction flask, add TAF-SM2 (646g) and potassium bicarbonate (441g) to free at 15-25°C for 5-10 hours, filter, drain, and control the temperature for 30- Concentrate to dryness under reduced pressure at 40°C, and ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com