Novel preparation process of tenofovir alafenamide fumarate
A technology of tenofovir alafenamide and hemifumaric acid, which is applied in the field of new preparation technology of tenofovir alafenamide hemifumarate, can solve the problem of high content of three pairs of diastereoisomers , Difficult to get effective control and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
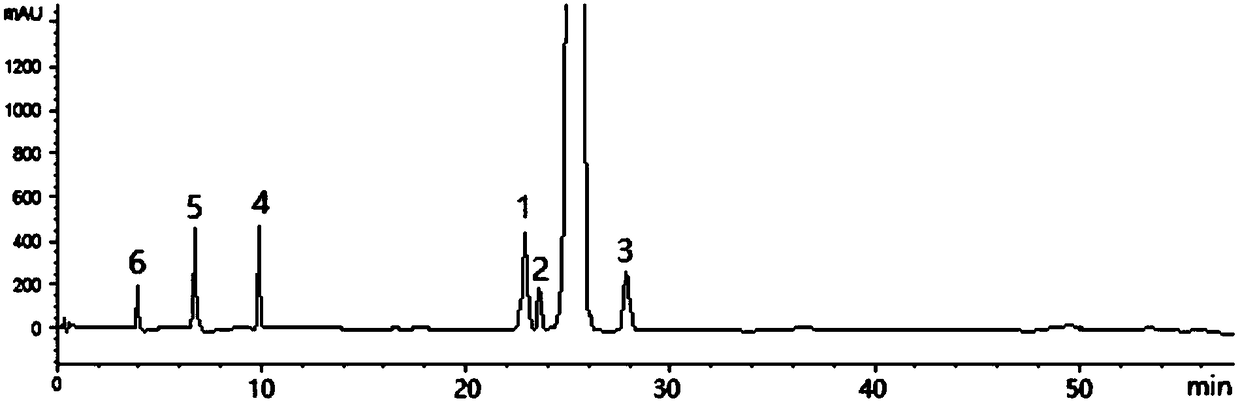
[0040] For the preparation method of tenofovir alafenamide, refer to US Patent Document US7803788. like figure 1 As shown, using the above-mentioned HPLC detection method to detect, in the tenofovir alafenamide crude product raw material used in the present invention, the content of tenofovir alafenamide is 88.28%, and the content of isomer I (peak 1) The content was 1.86%, the content of isomer II (peak 2) was 0.83%, and the content of isomer III (peak 3) was 1.25%. In addition, Impurity 1 (Peak 4), Impurity 2 (Peak 5) and Impurity 3 (Peak 6) could be resolved.
[0041] The novel preparation process of tenofovir alafenamide hemifumarate provided by the present invention can effectively remove non-enantiomers and other impurities, and the preparation of tenofovir alafenamide hemifumarate of the present invention The method includes the steps of:
[0042] (1) refining of tenofovir alafenamide
[0043] Add the crude product of tenofovir alafenamide into isobutanol, heat to d...
Embodiment 1
[0050] In the present embodiment, the preparation method of tenofovir alafenamide hemifumarate comprises the following steps:
[0051](1) refining of tenofovir alafenamide
[0052] Add 100 g of tenofovir alafenamide crude product (HPLC purity 88.28%) into 500 ml of isobutanol, heat to dissolve, then add 1000 ml of acetonitrile and n-hexane mixed solution (the volume ratio of acetonitrile and n-hexane is 6:4) After stirring evenly, the temperature was slowly lowered to 5° C., stirred and crystallized at 60 rpm for 2 hours, the filter cake was filtered and rinsed with cold acetonitrile, and then vacuum-dried to obtain the refined product of tenofovir alafenamide, with a yield of 86.3%.
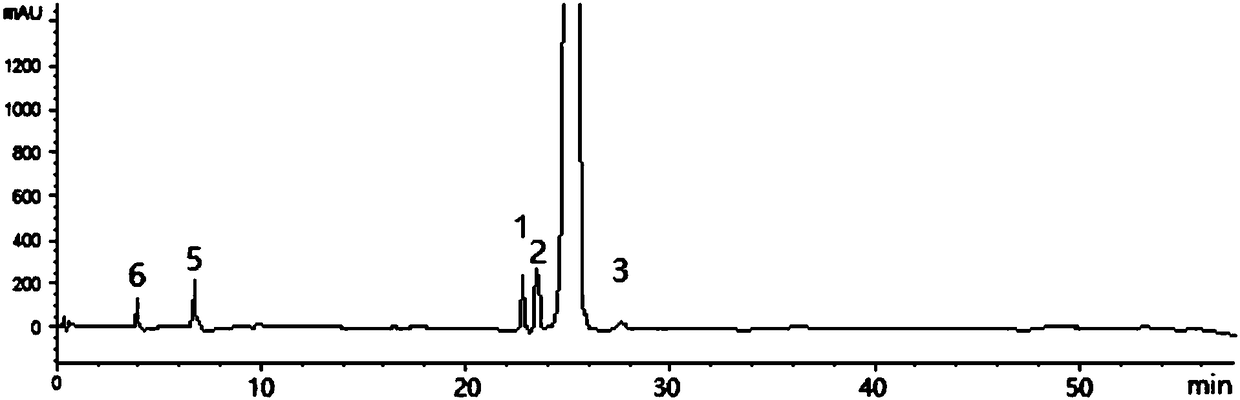
[0053] The HPLC detection result of tenofovir alafenamide refined product is as follows figure 2 Shown, the content of main peak tenofovir alafenamide is 98.13%, the content of isomer I (peak 1) is 0.52%, the content of isomer II (peak 2) is 0.97%, isomer III The content of (Peak 3) was 0.16%...
Embodiment 2
[0058] In the present embodiment, the preparation method of tenofovir alafenamide hemifumarate comprises the following steps:
[0059] (1) refining of tenofovir alafenamide
[0060] Add tenofovir alafenamide crude product 100g (HPLC purity 88.28%) into 800ml isobutanol, heat to dissolve, then add acetonitrile and n-hexane mixed solution 1500ml (the volume ratio of acetonitrile and n-hexane is 2:1) After stirring evenly, the temperature was slowly lowered to 5°C, stirred and crystallized at 40 rpm for 3 hours, the filtered cake was rinsed with cold acetonitrile and then vacuum-dried to obtain the refined product of tenofovir alafenamide with a yield of 81.6%.
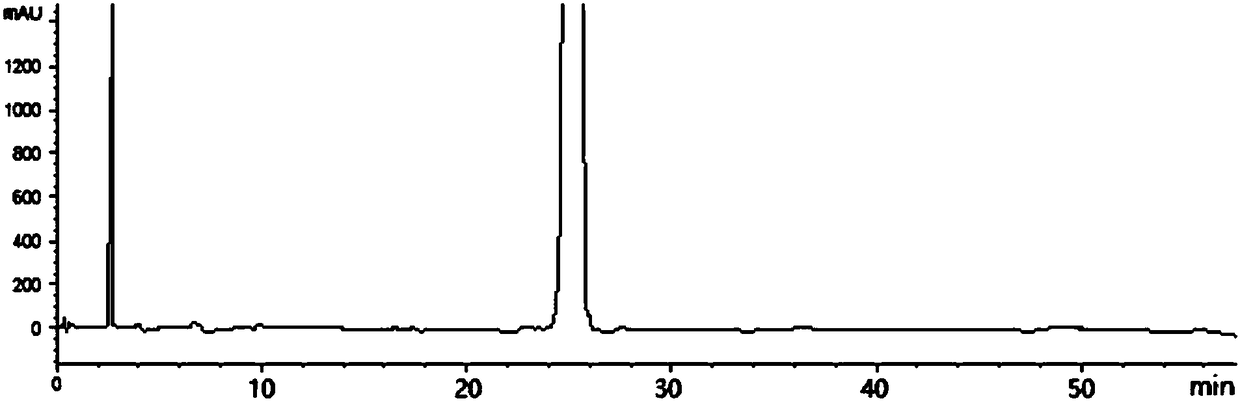
[0061] The HPLC detection result of tenofovir alafenamide refined product shows that the content of main peak tenofovir alafenamide is 98.06%, the content of isomer I (peak 1) is 0.48%, isomer II ( The content of peak 2) was 0.93%, and the content of isomer III (peak 3) was 0.26%.
[0062] (2) Preparation of tenofovir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com