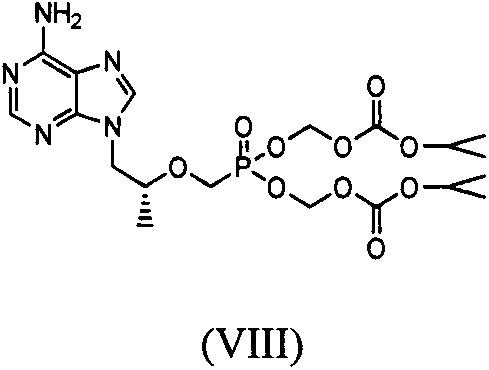
Tenofovir alafenamide fumarate impurity preparing method
A technology of tenofovir alafenamide fumarate and alafenamide, applied in the field of preparation of tenofovir alafenamide fumarate impurity, can solve the synthesis of tenofovir alafenamide fumarate impurity Issues such as insufficient literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19-
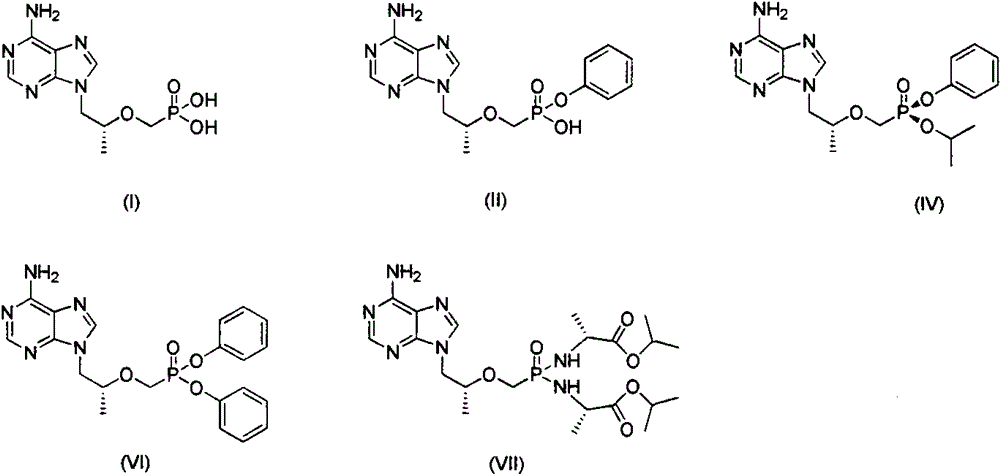
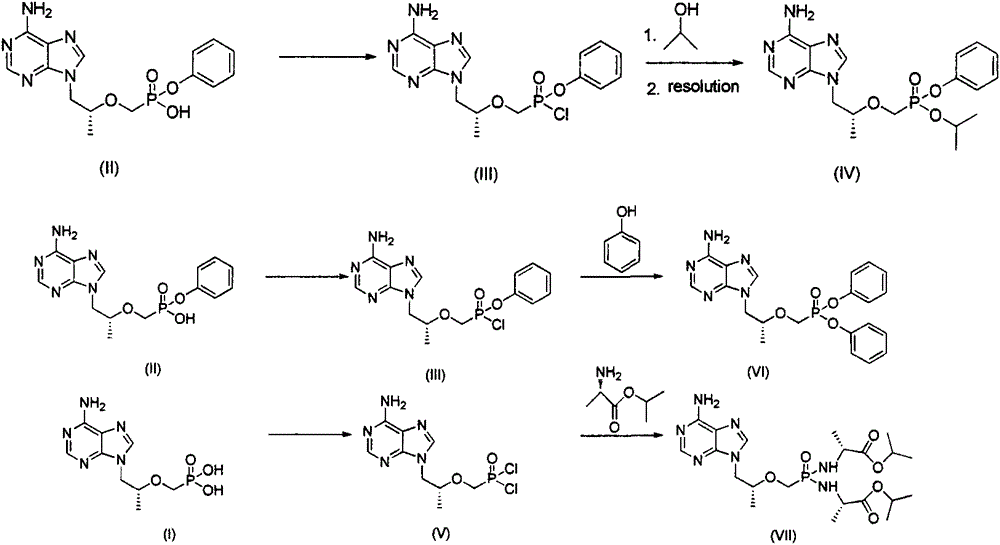
[0041] Preparation of Example 19-[(R)-2-[[(S)-[[(S)-1-isopropoxyphenoxyphosphinyl]methoxy]propyl]adenine (IV)
[0042] Put 1g of 9-[(R)-2-[[(phenoxyphosphinyl)methoxy]propyl]]adenine in a 50mL three-necked flask, add 15ml of acetonitrile and 0.64g of chlorinated chlorinated For sulfone, heat up to 70°C and stir for 2-3h. After the reaction is complete, concentrate, cool to -5°C, add 10ml of isopropanol, stir for 1h, concentrate, and the concentrate is separated by column chromatography to obtain 0.89g of light yellow oil, which is collected The rate is 80.1%.
[0043] 0.89 g of the yellow oil obtained above was resolved by preparative liquid phase to obtain 0.44 g of a white solid, with a yield of 49.4%.
[0044] Its structural identification data are as follows:
[0045] 1 H-NMR (400Mz, DMSO-d 6 )δ: 1.07 ~ 1.12 (d, 3H, CH 3 ), 1.14~1.21(m, 6H, 2×CH 3 ), 3.90~3.93(m, 1H, OCH), 3.94~4.03(m, 2H, CH 2 N), 4.14~4.30(m, 2H, OCH), 4.59~4.66(m, 1H, OCH(CH 3 ) 2 ), 6.62 (s, 2...
Embodiment 29-
[0047] Preparation of Example 29-[(R)-2-[[(S)-[two-(phenoxy)phosphinyl]methoxy]propyl]adenine (VI)
[0048] Put 1g of 9-[(R)-2-[[(phenoxyphosphinyl)methoxy]propyl]]adenine in a 50mL three-necked flask, add 15ml of acetonitrile and 0.64g of chlorinated chlorinated For sulfone, heat up to 70°C and stir for 2-3h. After the reaction is complete, concentrate, cool to -5°C, add 15mL of acetonitrile and 0.26g of phenol, stir for 1h, concentrate, and the concentrate is separated by column chromatography to obtain 0.85g of white solid. Yield 70.3%.
[0049] Its structural identification data are as follows:
[0050] 1 H-NMR (400Mz, DMSO-d 6 )δ: 1.26 ~ 1.28 (d, 3H, CH 3 ), 3.86~3.94 (m, 1H, CH 2 CHO), 4.11 ~ 4.20 (m, 2H, OCH 2 P), 4.35 ~ 4.39 (m, 2H, CH 2 N) 5.86 (s, 2H, NH 2 ), 7.06~7.36(m, 10H, 2×C 6 h 5 ), 7.89 (s, 1H, H-2), 8.34 (s, 1H, H-8).
[0051] ESI-MS(m / z): 442.10[M+H] + .
Embodiment 39-
[0052] Example 39-[(R)-2-[[two-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phosphinyl]methoxy]propyl]adenine (VII ) preparation
[0053] Put 1g of 9-[(R)-2-(phosphonomethoxy)propyl]adenine in a 50mL three-neck flask, add 15ml of acetonitrile and 0.64g of thionyl chloride at room temperature, heat and stir at 70°C for 2~ 3h, concentrated, cooled to -5°C, added 15mL of acetonitrile and 1.45g of L-alanine isopropyl ester, stirred for 1h, concentrated, the concentrate was separated by column chromatography to obtain 0.85g of white solid, yield 60.1%.
[0054] Its structural identification data are as follows:
[0055] 1 H-NMR (400Mz, DMSO-d 6 )δ: 1.14 ~ 1.15 (d, 3H, CH 3 ), 1.16~1.26 (d, 6H, 2×CH 3 ), 1.28~1.41 (d, 12H, 4×CH 3 ), 3.09(t, 1H, CH 2 CHO), 3.48~3.53(d, 2H, NH), 3.78~3.85(m, 2H, OCH 2 P), 3.89~3.97(m, 2H, CH 2 N), 4.83~4.87(m, 1H, CH(CH 3 ) 2 ), 5.05~5.09(m, 1H, CH(CH 3 ) 2 ), 6.06(s, 2H, NH 2 ), 8.09 (s, 1H, H-2), 8.32 (s, 1H, H-8).
[0056] ESI-MS(m / z): 51...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com