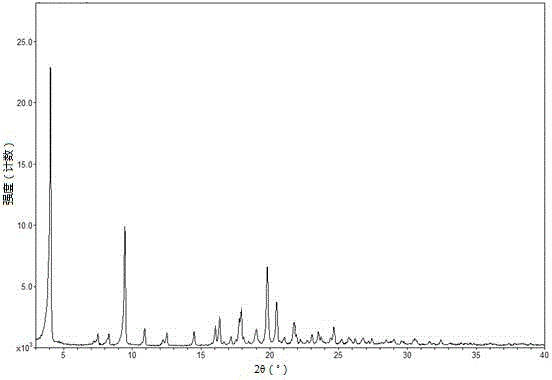
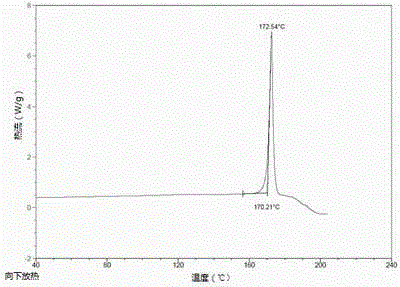
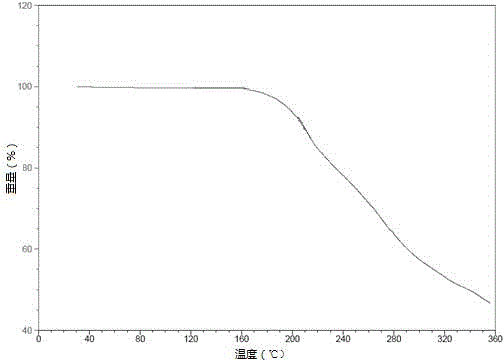
Tenofovir alafenamide semi-tartrate
A technology of tenofovir alafenamide and hemi-tartrate, which is applied in the field of tenofovir alafenamide hemi-tartrate, can solve the problems that tenofovir alafenamide has not been reported, and improve the purity and yield, improved chemical stability, and good crystallization properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of tenofovir alafenamide hemitartrate
[0036]Add 9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl] to the reactor equipped with stirrer Methoxy]propyl]adenine (5.00 g), tartaric acid (1.56 g), ethanol (30 ml) and acetonitrile (20 mL). The mixture was heated to 70°C-75°C to dissolve the solids. After filtration, the filtrate was cooled to 0°C-5°C over 4 hours. The temperature was maintained for 1-18 hours, and the resulting slurry was filtered and washed with 0.2 mL of acetonitrile (0°C-5°C). The solid was dried at 50° C. under vacuum to obtain 3.14 g of tenofovir alafenamide hemitartrate, yield: 48%, and its HPLC purity was 99.65% (area normalization method).
Embodiment 2
[0037] Example 2: Preparation of tenofovir alafenamide hemitartrate
[0038] Add 9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl] to the reactor equipped with stirrer Methoxy]propyl]adenine (5.00 g), L-tartaric acid (1.56 g), ethanol (30 ml) and acetonitrile (20 mL). The mixture was heated to 70°C-75°C to dissolve the solids. After filtration, the filtrate was cooled to 0°C-5°C over 4 hours. The temperature was maintained for 1-18 hours, and the resulting slurry was filtered and washed with 0.2 mL of acetonitrile (0°C-5°C). The solid was dried under vacuum at 50°C to obtain 6.30 g of tenofovir alafenamide hemitartrate, yield: 96%, and its HPLC purity was 99.75% (area normalized method).
Embodiment 3
[0039] Example 3: Preparation of tenofovir alafenamide hemitartrate
[0040] Add 9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl] to the reactor equipped with stirrer Methoxy]propyl]adenine (5.00 g), L-tartaric acid (1.56 g), methanol (30 ml) and diethyl ether (20 mL). The mixture was heated to 70°C-75°C to dissolve the solids. After filtration, the filtrate was cooled to 0°C-5°C over 4 hours. The temperature was maintained for 1-18 hours, and the resulting slurry was filtered and washed with 0.2 mL of acetonitrile (0°C-5°C). The solid was dried at 50° C. under vacuum to obtain 6.26 g of tenofovir alafenamide hemitartrate, yield: 95%, and its HPLC purity was 99.68% (area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com