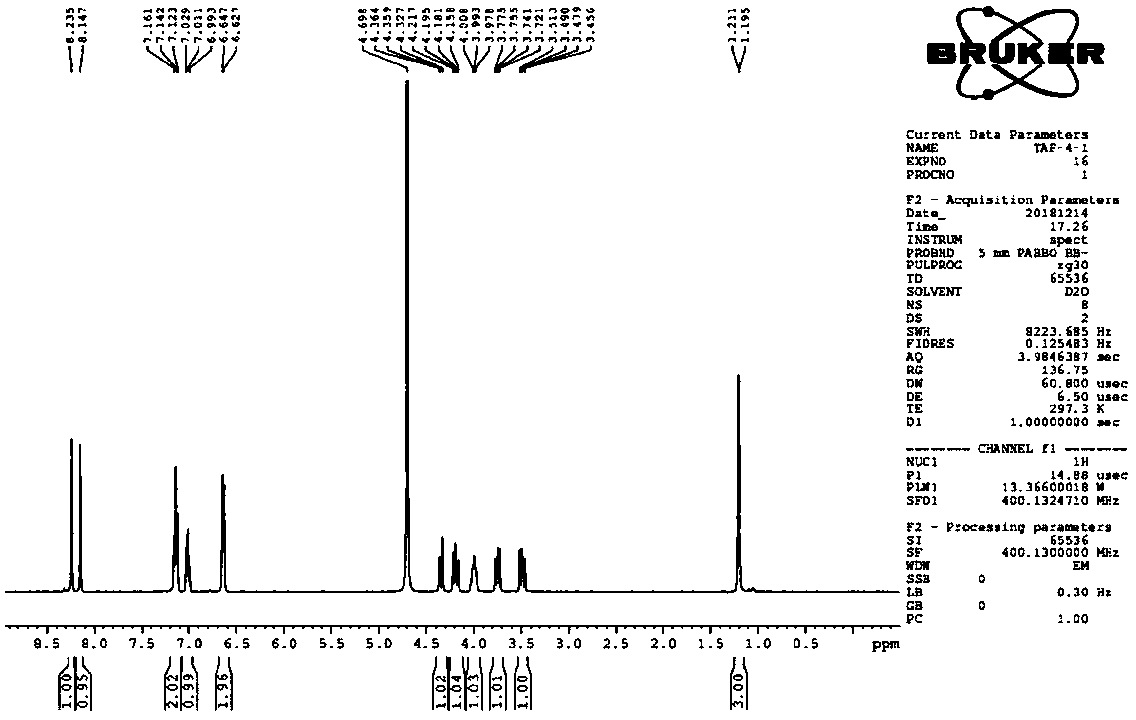
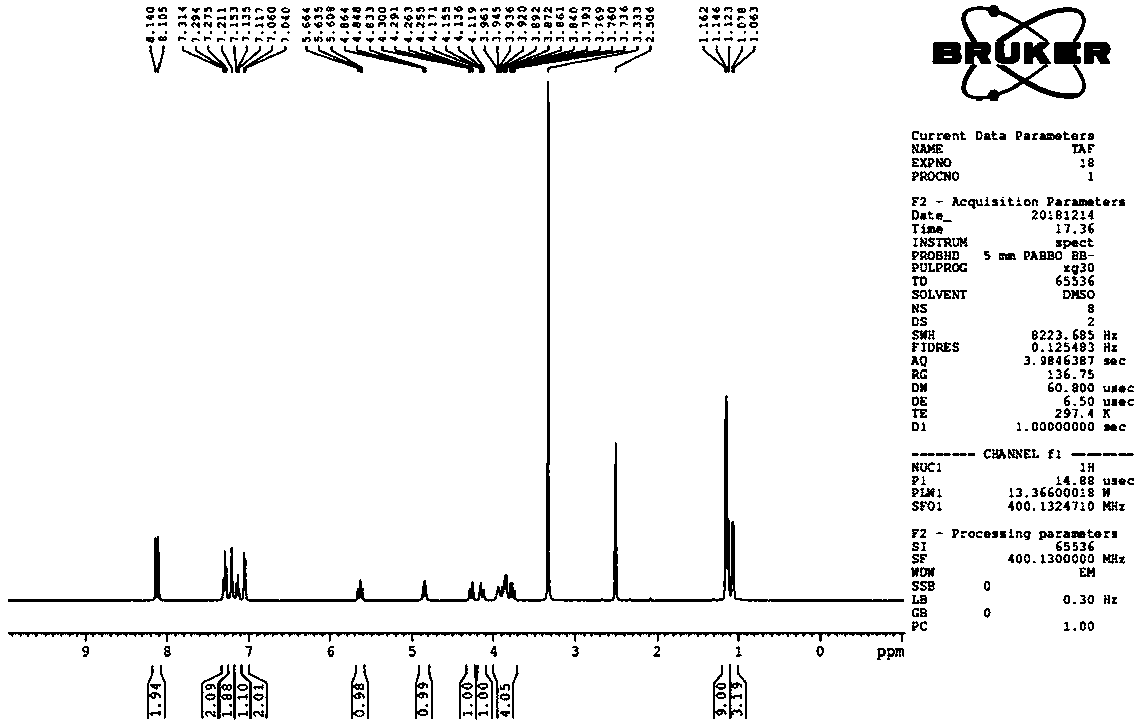
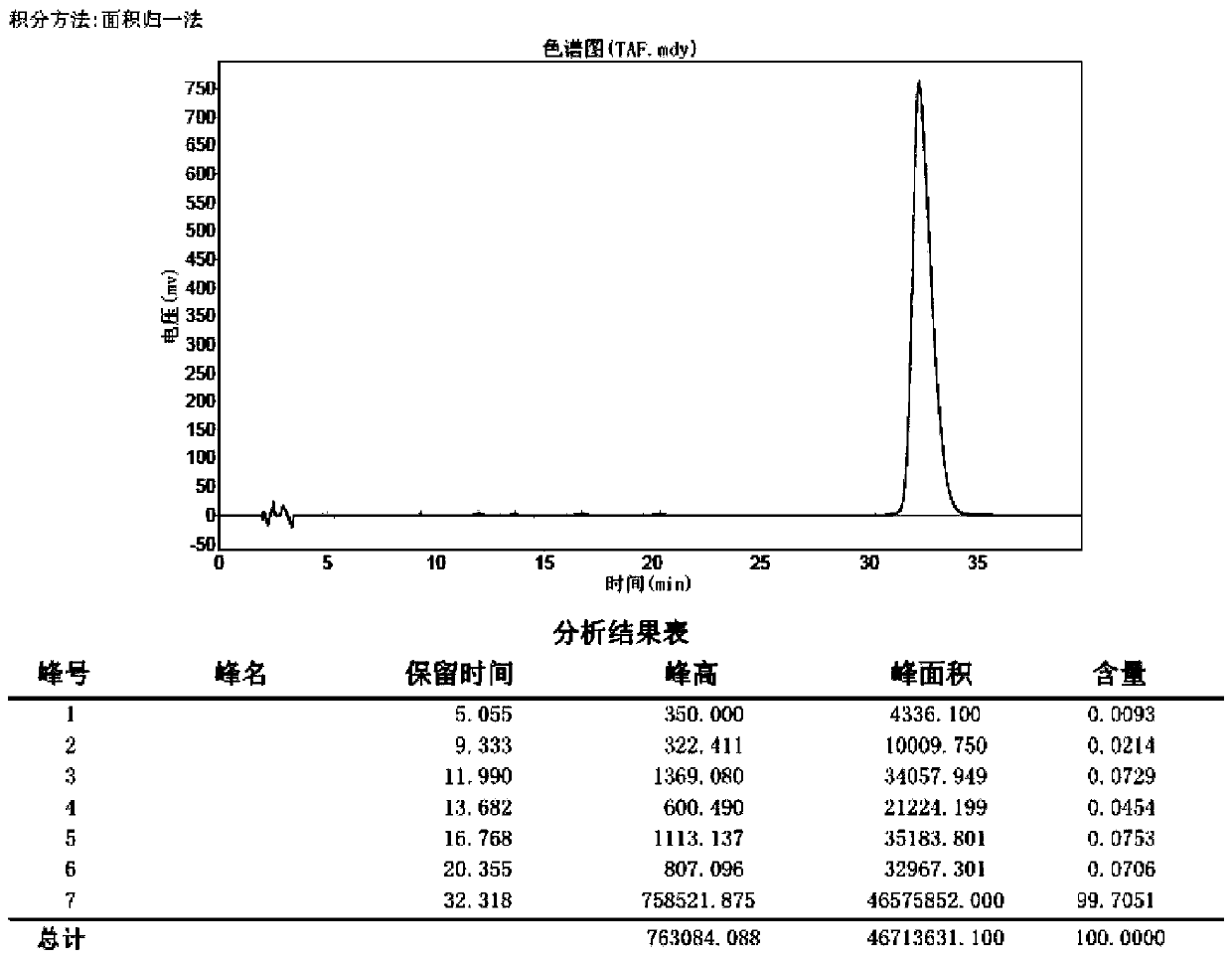
Efficient synthesis technology of tenofovir alafenamide
A technology of tenofovir alafenamide and synthesis process, which is applied in the field of high-efficiency synthesis process of antiviral drug tenofovir alafenamide, can solve the problems of difficult condition control, long operation time, cumbersome operation, etc. Achieve the effect of improving yield and purity, less consumption of raw materials, and high chemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of compound tenofovir monophenyl ester: anhydrous tenofovir (50.0g, 0.18mol) is stirred with pyridine (500ml) at room temperature to form a turbid solution, adding triphenyl phosphite (162.0g, 0.52mol) , the reaction temperature is 50-115°C; the temperature is raised gradually, the temperature rise range is 5-10°C / hour, and the reaction time is 6 hours. Cool to room temperature, filter, wash the filter cake with acetone (50ml), and dry the filter cake at 60-70°C for 4 hours to obtain 50.7 g of tenofovir monophenyl ester as a white solid, with a yield of 82.5%.
Embodiment 2
[0046] Add 1 equivalent of triphenyl phosphite to the filtered mother liquor in Example 1, and the other operations are the same, and 62.0 g of tenofovir monophenyl ester is prepared, with a yield of 98.0%.
Embodiment 3
[0048] The operation of Example 2 was repeated to obtain 61.4 g of tenofovir monophenyl ester, with a yield of 97.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com