Industrialized continuous production method of hemifumarate tenofovir alafenamide
A technology for tenofovir alafenamide and production method, which is applied in the field of industrialized continuous production of tenofovir alafenamide hemi-fumarate, can solve the problems of low yield and high equipment requirements, and can reduce production time , the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1, industrialized continuous production tenofovir alafenamide hemifumarate
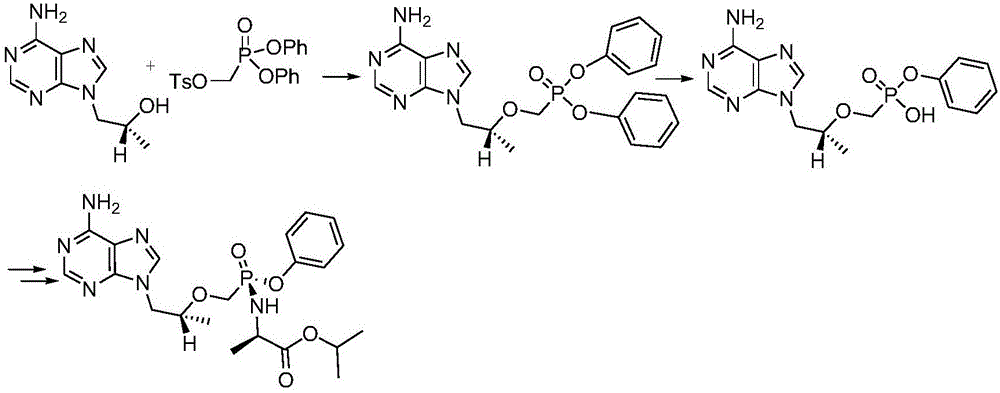
[0070] Step 1 (reaction flow chart sees Figure 5 ):
[0071] 120kg of acetonitrile was pumped into the 500L reactor, 20kg (69.6mol) of tenofovir was added, 32kg (103mol) of triphenyl phosphite, 8kg (65.5mol) of DMAP, 14kg (138mol) of triethylamine, and the temperature was slowly refluxed for 72 Hour. Cool down to 50°C and concentrate acetonitrile to dryness under reduced pressure, add 100 kg of purified water, extract three times with ethyl acetate, each time 60 kg, and keep the water layer. Acidify with hydrochloric acid to a pH value of 2.0-3.0, filter with a plate centrifuge, rinse the solid with 0.1M hydrochloric acid, rinse the solid with dichloromethane, filter until dry, and feed the solid with TAF-I M for later use.
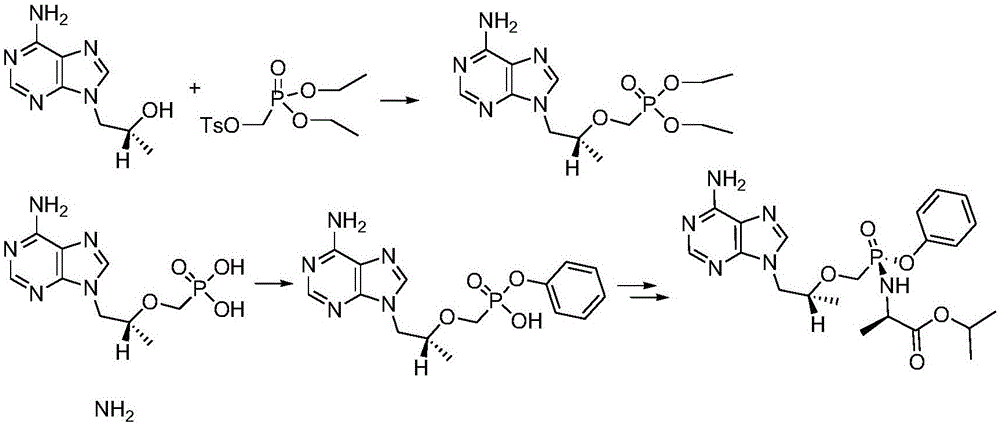
[0072] Step 2 (reaction flow chart sees Figure 6 ):
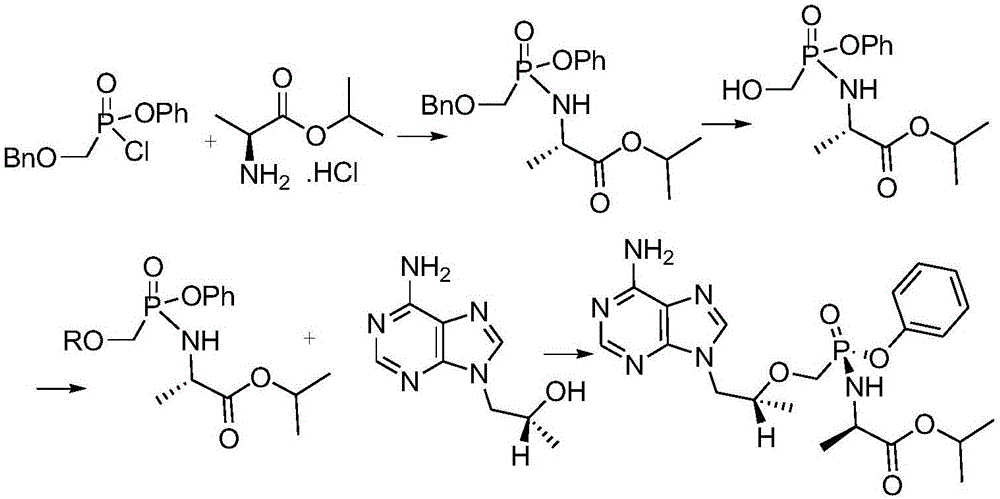
[0073] Into the 500L reactor I, suck 250kg of toluene, add the above-mentioned TAF-I M steam to rai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com