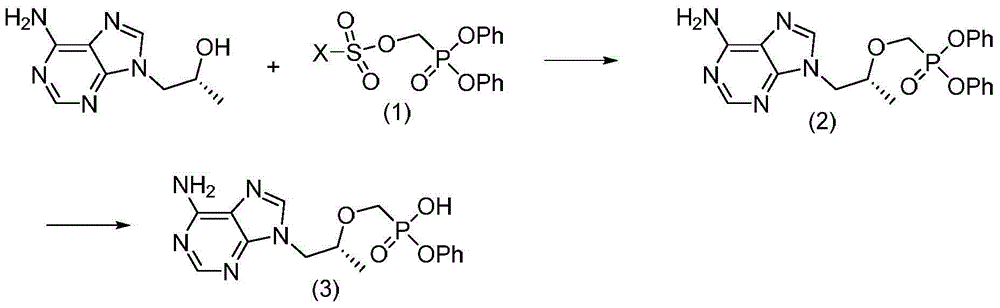
Synthetic process of key intermediate of hemifumarate tenofovir alafenamide
A technology of tenofovir alafenamide and synthesis process, which is applied in the field of synthesis process of key intermediate of tenofovir alafenamide hemifumarate, can solve the problem of unsuitable for industrial production, harsh hydrolysis conditions, no Illustrate examples and other issues to achieve the effects of low cost, easy access to raw materials, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of (R)-{[2-(6-amino-9H-purine-9-yl)-1-methylethoxy]methyl}phosphonic acid diphenyl ester
[0056]
[0057] Dissolve (R)-9-(2-hydroxypropyl)adenine (5g, 25.8mmol) in 25ml of N-methylpyrrolidone, add potassium tert-butoxide (2.88g, 25.8mmol) into it at room temperature, and stir After 1 hour, diphenyl p-toluenesulfonyloxyphosphonate (14 g, 33.5 mmol) was added, and the reaction was stirred at 25° C. for 12 hours. Add acetic acid (1.2g, 20.6mmol) dropwise again, and after stirring for 20 minutes, the reaction solution is poured into 100ml water, and off-white solids are precipitated, filtered, the filter cake is washed once with water, once with a small amount of ethanol, and dried to obtain 9.6g of solids. The yield was 85%.
[0058] The NMR data of product are as follows:
[0059] 1 H NMR (400MHz, d-DMSO): δ1.32(d,3H),3.35(dd,1H),3.55(dd,1H),4.5(m,2H),6.80–7.21(m,6H),7.31 –7.42(m,4H),8.26(s,1H),8.35(s,1H). 31 P NMR (162MHz, CDCl 3 ) δ18.6.
[0060] ...
Embodiment 2
[0068] Preparation of (R)-{[2-(6-amino-9H-purine-9-yl)-1-methylethoxy]methyl}phosphonic acid diphenyl ester
[0069] Dissolve (R)-9-(2-hydroxypropyl)adenine (5g, 25.8mmol) in 25ml of N-methylpyrrolidone, add sodium hydrogen (1g, 25.8mmol, 60%) at room temperature, stir for 1 After one hour, diphenyl p-toluenesulfonyloxymethylphosphonate (14 g, 33.5 mmol) was added, and the reaction was stirred at 25° C. for 12 hours. Then add acetic acid (1.2g, 20.6mmol) dropwise, and after stirring for 20 minutes, the reaction solution is poured into 100ml water, and an off-white solid is precipitated, filtered, the filter cake is washed once with water, once with a small amount of ethanol, and dried to obtain 8.1g of solid, producing The rate is 72%.
[0070] Preparation of (R)-{[2-(6-amino-9H-purine-9-yl)-1-methylethoxy]methyl}phosphonic acid monophenyl ester
[0071] Preparation of (R)-{[2-(6-amino-9H-purine-9-yl)-1-methylethoxy]methyl}phosphonic acid diphenyl ester (8.1 g, 18.5 mmol) wa...
Embodiment 3
[0073] Preparation of (R)-{[2-(6-amino-9H-purine-9-yl)-1-methylethoxy]methyl}phosphonic acid diphenyl ester
[0074] Dissolve (R)-9-(2-hydroxypropyl)adenine (10g, 51.6mmol) in 40ml of N,N-dimethylformamide, and add magnesium tert-butoxide (7g, 41.3mmol) at room temperature After stirring for 1 hour, diphenyl p-toluenesulfonyloxymethylphosphonate (21.6 g, 51.6 mmol) was added, and the mixture was stirred and reacted at 75° C. for 8 hours. Add acetic acid (4.9g, 82.6mmol) dropwise, and after stirring for 20 minutes, pour the reaction solution into 250ml of water and 100ml of dichloromethane, extract and separate the layers, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, and filter , the filtrate was spin-dried under reduced pressure to obtain 17 g of solid, with a yield of 75%.
[0075] Preparation of (R)-{[2-(6-amino-9H-purine-9-yl)-1-methylethoxy]methyl}phosphonic acid monophenyl ester
[0076] Preparation of (R)-{[2-(6-amino-9H-purine-9-yl)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com