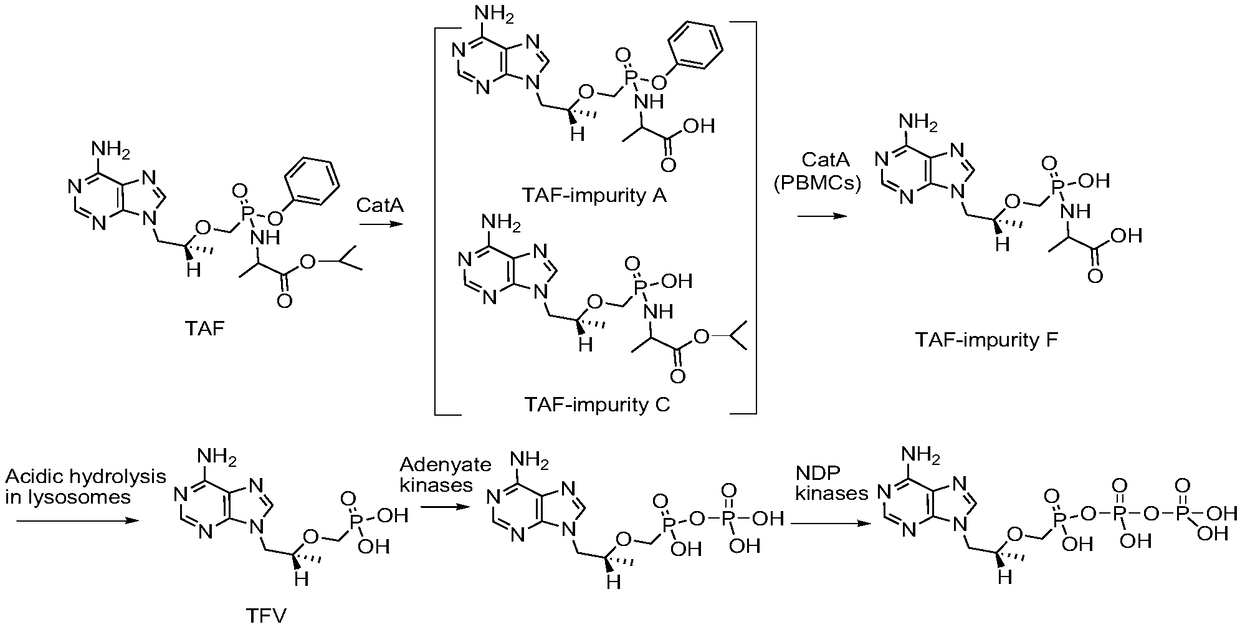
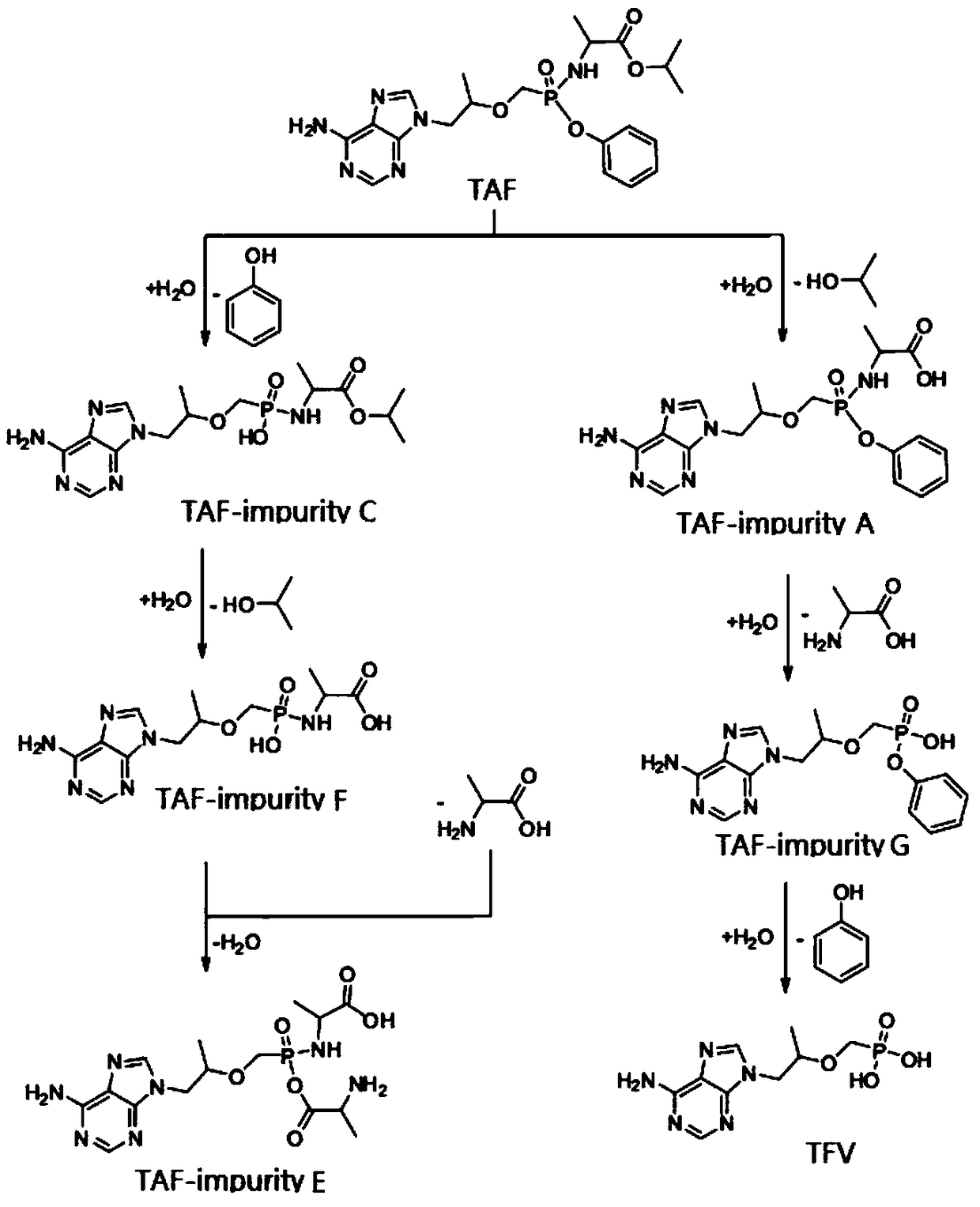
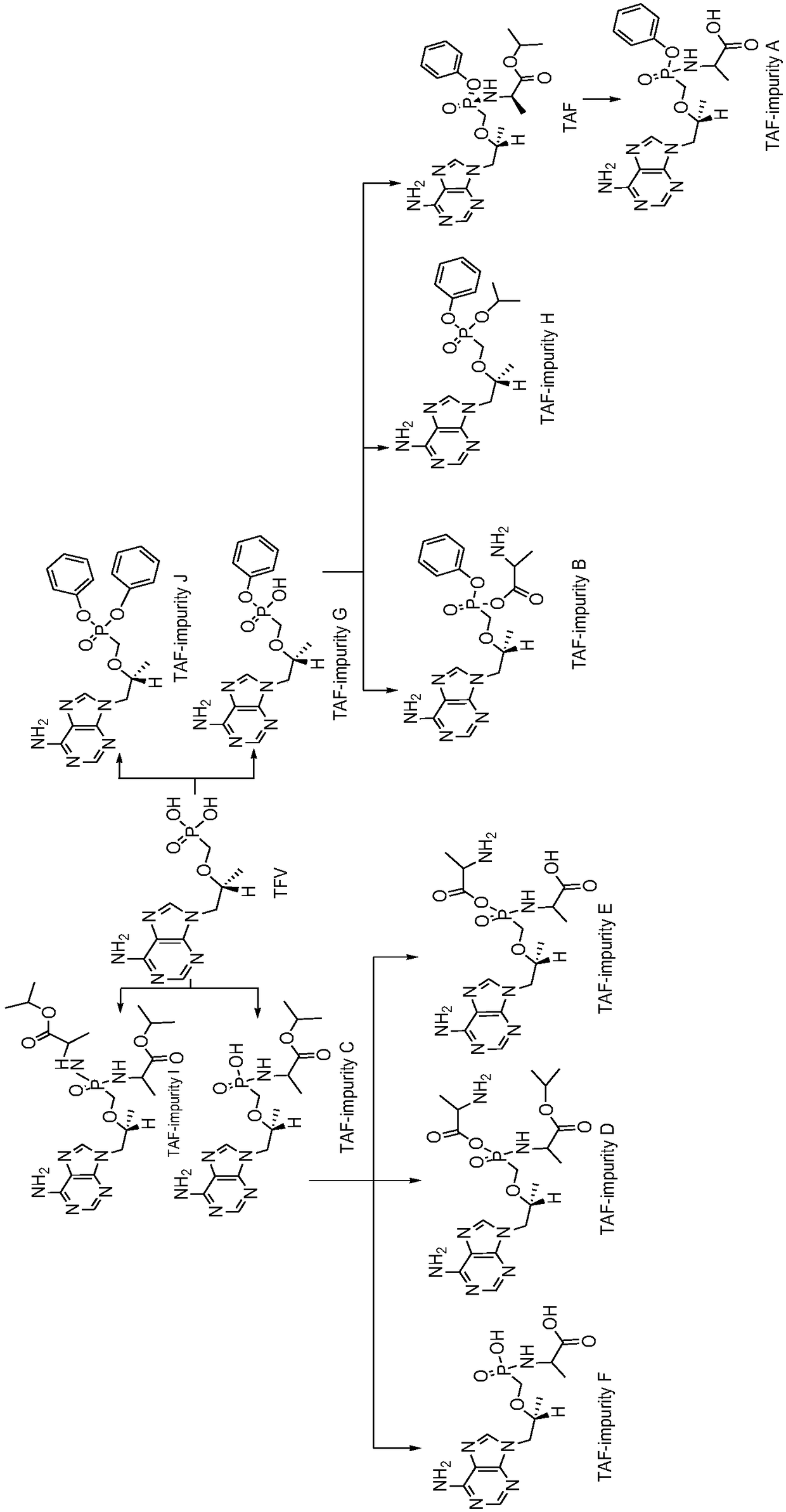
Synthesis method of potential impurities in production of tenofovir alafenamide hemifumarate
A technology for amides and compounds, applied in the field of synthesis of potential impurities, can solve problems such as no literature reports, and achieve the effect of improving production technology and improving product internal control quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1, synthetic TAF-impurity A
[0065]
[0066] Add 120ml of tetrahydrofuran to a 500ml reaction flask, add 9-[(R)-2-[[(S)-[[(S)-1-(tert-butyloxycarbonyl)ethyl]amino]phenoxyoxy Phosphino]methoxy]propyl]adenine 10g was added dropwise to a tetrahydrofuran solution of 2 mol / L sodium bis(trimethylsilyl)amide, the reaction was monitored by TLC, and the reaction was quenched by acetic acid. Tetrahydrofuran was concentrated under reduced pressure and purified by column chromatography with V dichloromethane:V methanol at 2:1 to obtain a white solid impurity 9-[(R)-2-[[(S)-[[(S)-1-propane Acid] amino] phenoxyphosphinyl] methoxy] propyl] adenine (TAF-impurity A) 6.0 g, yield 68%, purity greater than 98%.
[0067] Structural Confirmation Data:
[0068] TAF-impurity A mass spectrum data: m / z M+1:435.2[C18H23N6O5P+].
[0069] NMR data: H NMR (400MHz, d6-DMSO)
[0070] δ10.92(brs,1H),δ8.22(s,1H),δ8.16(s,1H),δ7.36-7.32(t,4H),δ7.18-7.16(t,1H),
[0071] δ7.09-7.07(d,2H)...
Embodiment 2
[0073] Embodiment 2, synthetic TAF-impurity B
[0074]
[0075] Add 500ml of toluene to a 1L reaction flask, add 50g of 9-[(R)-2-[[(phenoxyphosphinyl)methoxy]propyl]adenine and raise the temperature to 90°C to reflux and divide water until no Obvious moisture comes out, slowly add 30g of thionyl chloride dropwise, keep warm at 80°C for 20 hours, concentrate under reduced pressure until no obvious fraction comes out, cool down to room temperature under nitrogen protection, add 200ml of dichloromethane, stir and disperse, transfer to the dropping funnel stand-by.
[0076] Add 2000ml of dichloromethane into a 5L reaction flask, add 100g of N-benzyloxycarbonyl-L-alanine-N-hydroxysuccinimide ester, cool to -15°C under nitrogen protection, and slowly add chloride , DBU was added dropwise, and the reaction was completed at room temperature for 20 hours. Add 10% aqueous solution of sodium dihydrogen phosphate, let the saturated sodium chloride solution stand for stratification, c...
Embodiment 3
[0081] Embodiment 3, synthesis TAF-impurity C, TAF-impurity D, TAF-impurity E
[0082]
[0083] Add 500ml of toluene to a 1L reaction flask, add 50g of tenofovir and raise the temperature to 90°C to reflux and divide the water until no obvious water comes out, slowly add 30g of thionyl chloride dropwise, keep warm at 80°C for 20 hours, and decompress Concentrate until no obvious fraction comes out, then cool down to room temperature under nitrogen protection, add 200ml of dichloromethane, stir and disperse for later use.
[0084] Add 500ml of dichloromethane into a 3L reaction flask, add 32g of L-alanine isopropyl hydrochloride and 40g of potassium bicarbonate, add 50g of anhydrous sodium sulfate to free at room temperature for 4 hours, filter and drain, under nitrogen protection Cool to -15°C, slowly add triethylamine and the above-mentioned chlorinated compound, keep warm for reaction after adding, and monitor the completion of the reaction by TLC. Add 10% aqueous soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com