Medicinal solid preparation of tolvaptan and preparation method
A solid preparation, tolvaptan technology, applied in the field of pharmaceutical preparations, can solve the problems of large material loss, many types of organic solvents, poor control of the particle size of amorphous substances, etc., and achieve high bioavailability and good bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
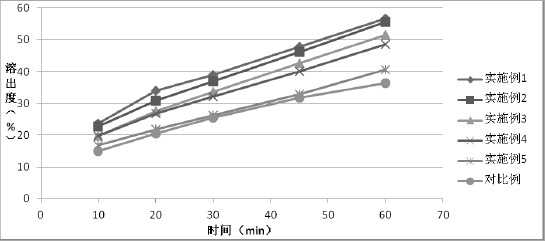
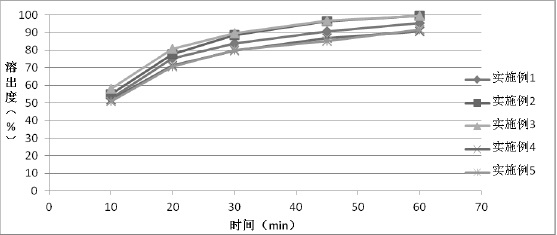
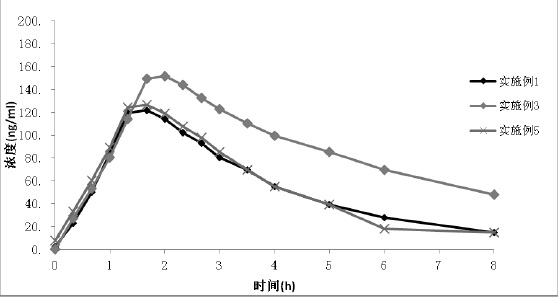
Examples
Embodiment 1
[0032] Embodiment 1 Preparation of Tolvaptan Solid Formulation Tablet
[0033] Mix 1:0.5 tolvaptan and hydroxypropyl cellulose, add absolute ethanol and dichloromethane to dissolve completely, carry out spray drying, adjust the air inlet temperature at 130 degrees, spray pressure at 0.06MPa, liquid delivery speed 20rpm, air volume 0.3m 3 / h, obtain solid dispersion amorphous powder, measure that its particle diameter D90 is 21 microns.
[0034] Adopt above-mentioned process gained spray-dried amorphous powder, carry out tolvaptan medicine solid preparation preparation by fluidized bed process, obtain tolvaptan tablet, prescription is as follows:
[0035] Table 1 Example prescription
[0036]
[0037] Preparation method: Weigh microcrystalline cellulose, corn starch, lactose and tolvaptan solid dispersion powder, disperse through a 60-mesh sieve; add the binder solution prepared by hydroxypropyl cellulose, and perform one-step fluidized bed granulation and drying; then ad...
Embodiment 2
[0038] Example 2 Preparation of Tolvaptan Solid Formulation Tablet
[0039] Mix 1:0.5 tolvaptan and hydroxypropyl cellulose, add absolute ethanol and dichloromethane to dissolve completely, and carry out spray drying; adjust the air inlet temperature at 130 degrees, spray pressure at 0.045MPa, liquid delivery speed 25rpm, air volume 0.3m 3 / h, obtain solid dispersion amorphous powder, measure that its particle diameter D90 is 39 microns.
[0040] The spray-dried amorphous powder obtained in the above process is used to prepare the tolvaptan drug solid preparation through a fluidized bed process to obtain tolvaptan tablets. The granulation prescription process is the same as in Example 1.
Embodiment 3
[0041] Example 3 Preparation of Tolvaptan Solid Formulation Tablet
[0042] Mix 1:0.5 tolvaptan and hydroxypropyl cellulose, add absolute ethanol and dichloromethane to dissolve completely, and carry out spray drying; adjust the air inlet temperature at 130 degrees, the spray pressure at 0.03MPa, and the liquid delivery speed 25rpm, air volume 0.3m 3 / h, obtain solid dispersion amorphous powder, measure that its particle diameter D90 is 56 microns.
[0043] The spray-dried amorphous powder obtained in the above process is used to prepare the tolvaptan drug solid preparation through a fluidized bed process to obtain tolvaptan tablets. The granulation prescription process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com