Preparation process of compound valsartan amlodipine solid preparation
A technology for solid valsartan amlodipine and amlodipine besylate, applied in the field of medicine, can solve the problems of short shelf life, low dissolution rate, low density of raw materials, etc., and achieves good stability, simple operation and high dissolution rate. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
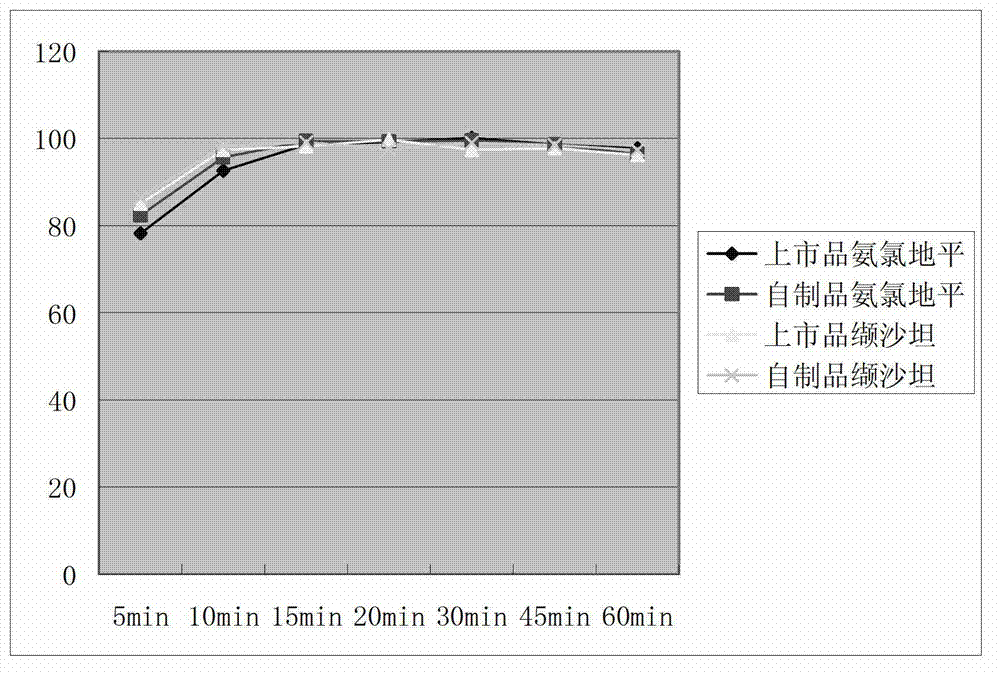
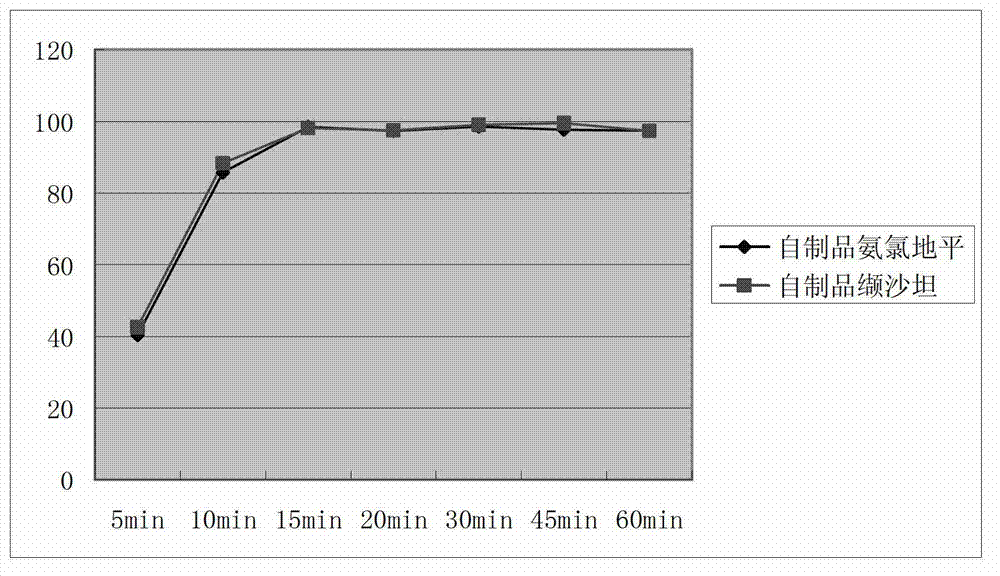
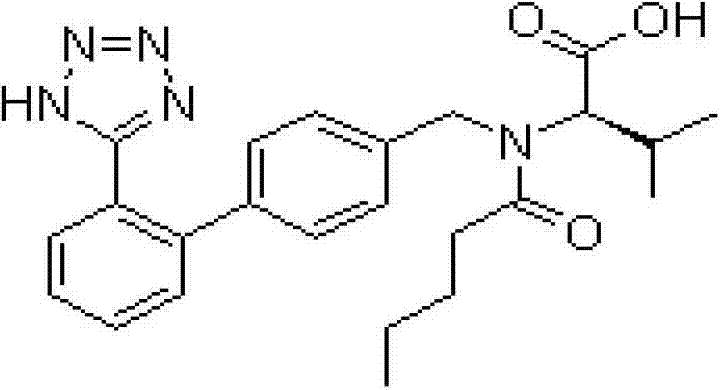
Image
Examples
Embodiment 1
[0056] Embodiment 1, tablet prescription and technology:
[0057]
[0058] Process:
[0059] 1) Take the valsartan raw material, pulverize it, pass through a 200-mesh sieve, and set aside.
[0060] 2) Take an appropriate amount of 50% ethanol aqueous solution, add the prescribed amount of amlodipine besylate, stir to dissolve. As an adhesive, set aside.
[0061] 3) Take valsartan and microcrystalline cellulose in a fluidized bed granulator, turn on the hot air, control the temperature at 75±5°C, boil the material, and dissolve 50% ethanol with amlodipine besylate The aqueous solution is atomized through a spray gun, sprayed into the mixed material containing valsartan and microcrystalline cellulose, and granulated. After granulation, continue to keep hot air for about 20 minutes, and dry the obtained granules.
[0062] 4) Take out the dry granules from the fluidized bed granulator, size the granules, calculate the yield, add crospovidone, micronized silica gel, magnesium...
Embodiment 2
[0066] Embodiment 2, capsule prescription and technology:
[0067]
[0068] Process:
[0069] 1) Take the valsartan raw material, pulverize it, pass through a 200-mesh sieve, and set aside.
[0070] 2) Take an appropriate amount of 50% ethanol aqueous solution, add the prescribed amount of amlodipine besylate, stir to dissolve. As an adhesive, set aside.
[0071] 8) Take valsartan and microcrystalline cellulose in a fluidized bed granulator, turn on the hot air, control the temperature at 75±5°C, boil the material, and dissolve 50% ethanol with amlodipine besylate The aqueous solution is atomized through a spray gun, sprayed into the mixed material containing valsartan and microcrystalline cellulose, and granulated. After granulation, continue to keep hot air for about 20 minutes, and dry the obtained granules.
[0072] 3) Take out the dry granules from the fluidized bed granulator, granulate, calculate the yield, add crospovidone, micronized silica gel, magnesium steara...
Embodiment 3
[0075] Embodiment 3, tablet
[0076]
[0077] Preparation method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com