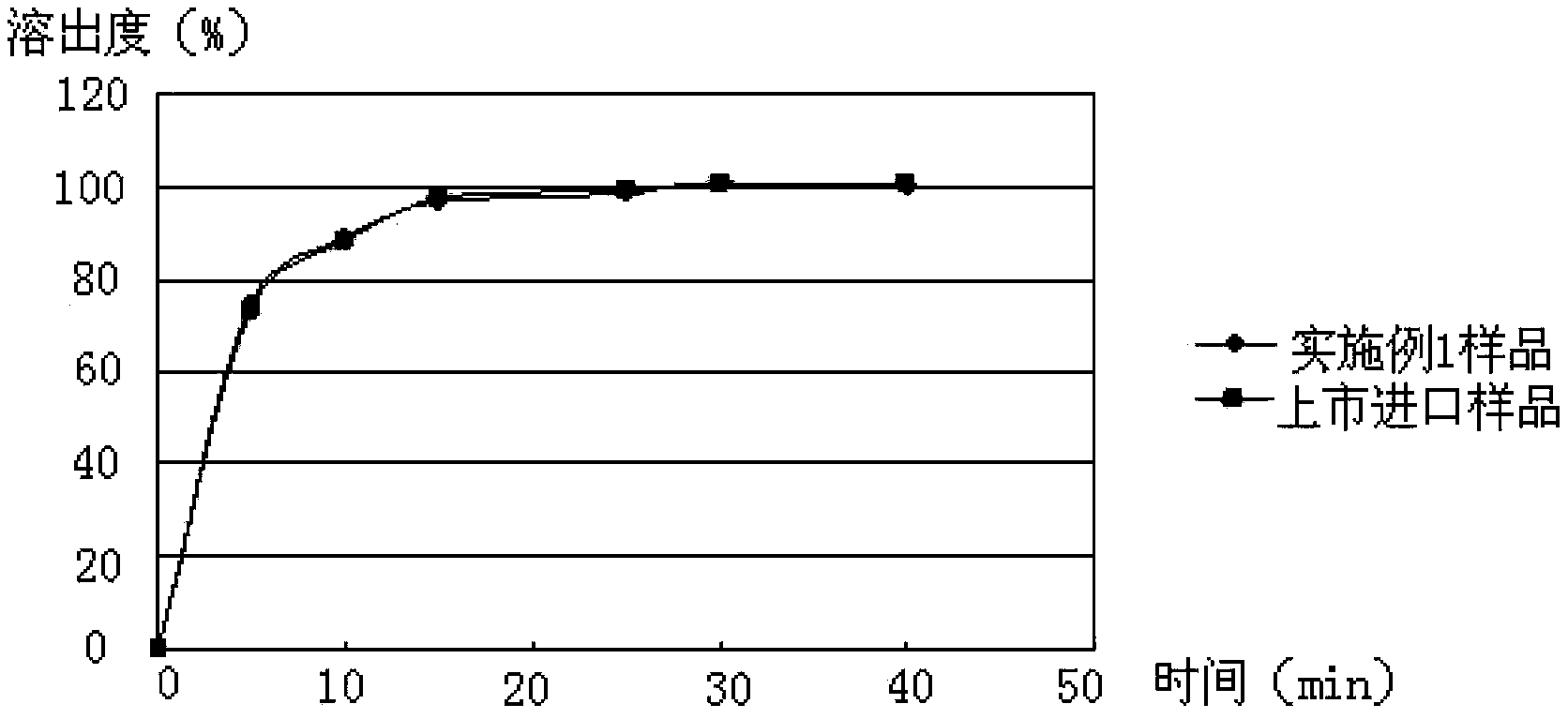
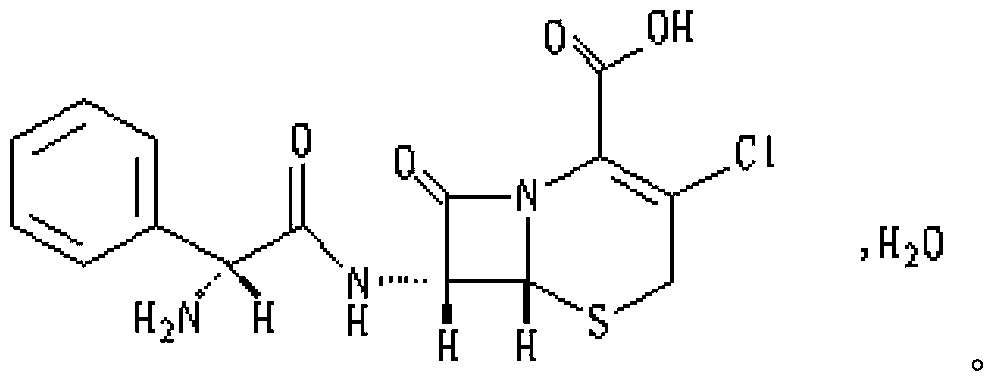
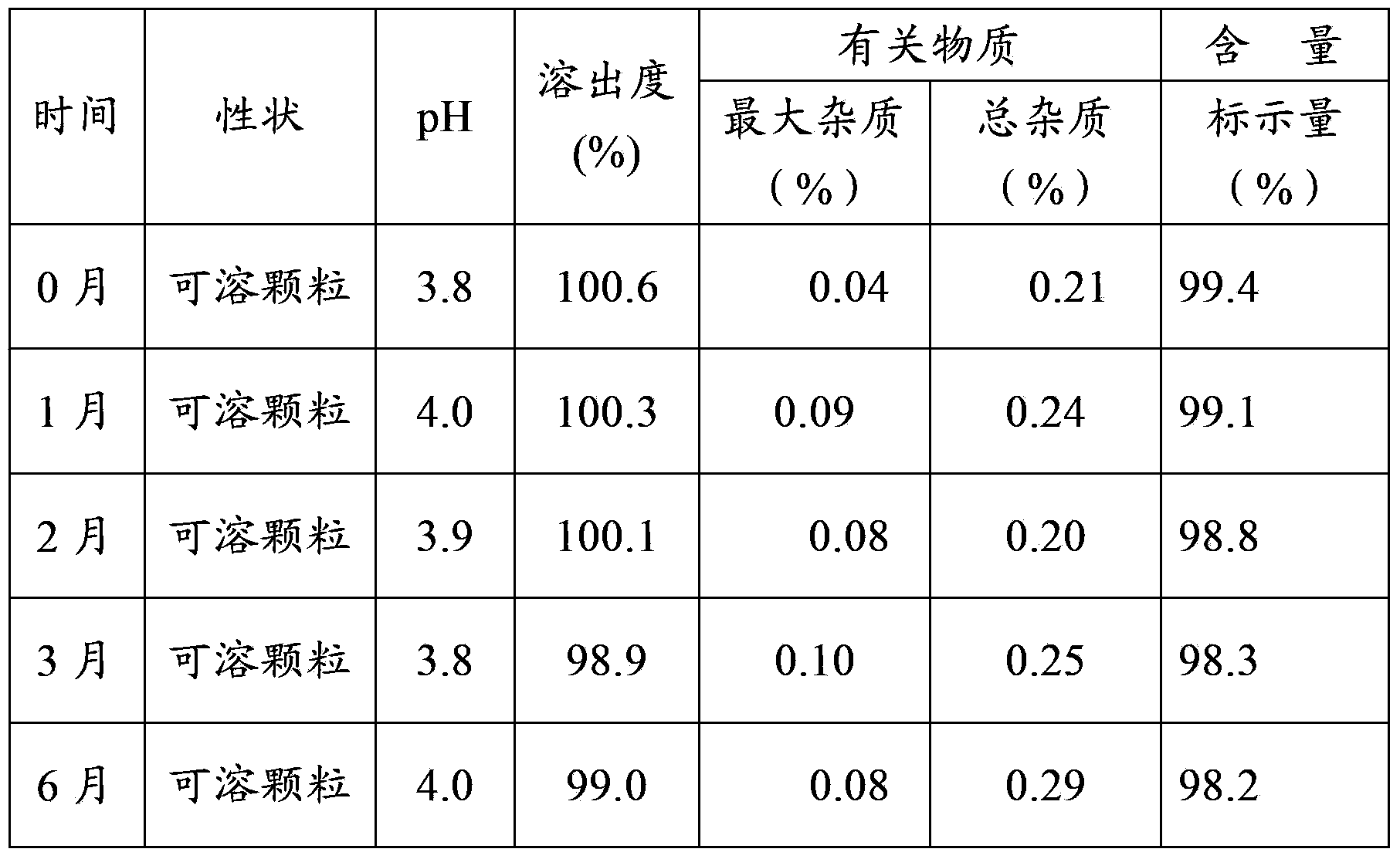
Medicinal composition containing cefaclor particles, and preparation method and application thereof
A technology of Cefaclor granules and Cefaclor, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, antibacterial drugs, etc., can solve problems such as no reports of Cefaclor, and achieve the goal of reducing hydrolysis and thermal damage degree, good water solubility, and reduce the effect of operation links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 The technique that fluidized bed of the present invention prepares cefaclor granule
[0042] (1) Cefaclor granule formula: see Table 1.
[0043] Table 1. Cefaclor Granule Formula
[0044] component name parts by weight role in preparation Cefaclor 10 Main drug sucrose 80 fillers, flavoring agents dextrin 60 filler 5% sodium carboxymethyl cellulose solution 20 Adhesive sodium citrate 10 stabilizer Solid Orange Flavor 5 Taste masking agent
[0045] (2) Preparation process:
[0046]Pass the above-mentioned raw materials and auxiliary materials through 80-mesh sieve respectively, weigh the remaining components except the adhesive according to the prescription in Table 1, place them at the bottom of the fluidized bed container, and control the air intake to 1500M 3 / hour, the inlet air temperature is controlled at 55°C, keep the mixed material for 15 minutes, raise the inlet air temperature t...
Embodiment 2
[0048] Embodiment 2 The technique that fluidized bed of the present invention prepares cefaclor granule
[0049] (1) Cefaclor granule formula: see Table 2.
[0050] Table 2. Cefaclor Granule Formula
[0051] component name parts by weight role in preparation Cefaclor 10 Main drug lactose 40 fillers, flavoring agents corn dextrin 100 filler 3% crospovidone solution 20 Adhesive Sodium acetate 10 stabilizer Solid Orange Flavor 5 Taste masking agent
[0052] (2) Preparation process:
[0053] Pass the above-mentioned raw materials and auxiliary materials through 80 mesh sieves respectively, weigh the remaining components except the binder according to the prescription in Table 2, place them at the bottom of the fluidized bed container, and control the air intake to 1000M 3 / hour, the air inlet temperature is controlled at 35°C, keep the mixed material for 15 minutes, raise the air inlet temperature to 60°C, add ...
Embodiment 3
[0055] Embodiment 3 The technique that fluidized bed of the present invention prepares cefaclor granule
[0056] (1) Cefaclor granule formula: see Table 3.
[0057] Table 3. Cefaclor Granule Formula
[0058] component name parts by weight role in preparation Cefaclor 10 Main drug Mannitol 40 fillers, flavoring agents dextrin 100 filler 5% crospovidone solution 20 Adhesive Sodium hydrogen phosphate 10 stabilizer xylitol 5 Taste masking agent
[0059] (2) Preparation process:
[0060] Pass the above-mentioned raw materials and auxiliary materials through 80-mesh sieve respectively, weigh the remaining components except the binder according to the prescription in Table 3, place them at the bottom of the fluidized bed container, and control the air intake to 1000M 3 / hour, the air inlet temperature is controlled at 40°C, keep the mixed material for 15 minutes, raise the air inlet temperature to 80°C, add the ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com