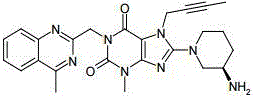
Linagliptin active component-containing composition and preparation method thereof
A technology of active ingredient and composition, applied in the field of linagliptin tablet composition and preparation, can solve problems such as complex process, achieve simple operation and reduce exposure links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
8000 tablets
[0029] Process:
[0030] Preparation of raw material suspension: Weigh 1 / 6 of the prescription amount of mannitol, dissolve it in 800 g of water, and then suspend the raw material.
[0031] Adhesive preparation: Weigh the prescription amount of povidone K90 and dissolve it in an appropriate amount of water to prepare a 6% povidone K90 aqueous solution.
[0032] First heat the fluidized bed granulator until the parameters are stable (temperature: 60℃), then put the remaining mannitol, pregelatinized starch and corn starch into the fluidized bed granulator, mix for 10 minutes, and spray the raw materials into suspension After spraying, spray the adhesive (according to the state of the particles, control the spray stop time), after drying, the particle moisture is controlled at 2-4%.
Embodiment 2
8000 tablets
[0035] Process:
[0036] Adhesive preparation: Weigh the prescription amount of povidone K90 and dissolve it in an appropriate amount of water to prepare a 3% povidone K90 aqueous solution, and suspend the raw materials in it.
[0037] First heat the fluidized bed granulator until the parameters are stable (temperature: 60℃), then put the remaining mannitol, pregelatinized starch and corn starch into the fluidized bed granulator, mix for 10 minutes, and spray the adhesive (According to the particle status, control the spray stop time), after drying, the particle moisture is controlled at 2-4%.
Embodiment 3
8000 tablets
[0040] Process:
[0041] Adhesive preparation: Weigh the prescription amount of copovidone and dissolve it in water to prepare a 15% copovidone aqueous solution, and suspend the raw materials in it.
[0042] First heat the fluidized bed granulator until the parameters are stable (temperature: 60℃), then put the remaining mannitol, pregelatinized starch and corn starch into the fluidized bed granulator, mix for 10 minutes, and spray the adhesive (According to the particle status, control the spray stop time), after drying, the particle moisture is controlled at 2-4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com