Pellet medicine combination containing valsartan, amlodipine and hydrochlorothiazide
A technology of hydrochlorothiazide and amlodipine, which is applied in the field of medicine and can solve problems such as increased absorption, prolonged residence time, and incompleteness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
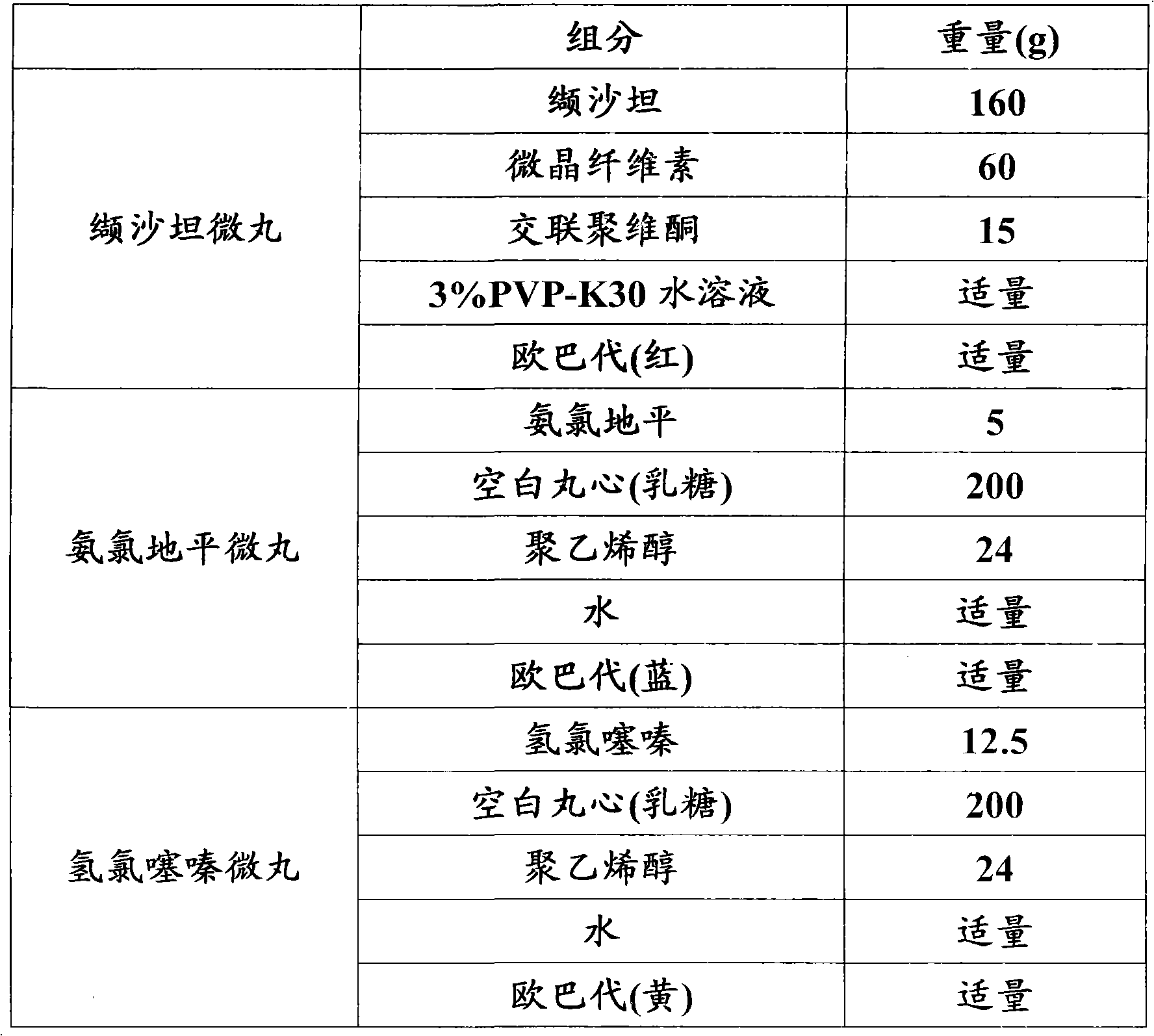
[0090] Embodiment 1: Valsartan amlodipine hydrochlorothiazide pellet capsule (160 / 5 / 12.5mg)
[0091]
[0092] Preparation:
[0093] Valsartan pellets: Pass valsartan, microcrystalline cellulose, and crospovidone through an 80-mesh sieve, mix well, make a soft material with 3% PVP-K30 aqueous solution, and put the soft material in an extrusion-spheronizer Prepare drug-containing micropills, pass the drug-containing micropills through a 16-40 mesh sieve, place in a fluidized bed and coat with Opadry until the weight of the micropills increases by 1.5-3%, dry until the water content is less than 1%, pass through 16 -40 mesh sieves, get valsartan pellets, standby;
[0094] Amlodipine pellets: Weigh the prescribed amount of amlodipine and polyvinyl alcohol and disperse them in water, stir until the solution becomes a uniform suspension; put the blank pill core in the fluidized bed, spray the suspension to apply the medicine, spray After the solution is completed, spray Opadry ...
Embodiment 2
[0098]Embodiment 2: Valsartan, levamlodipine, hydrochlorothiazide pellet capsules (160 / 2.5 / 12.5mg)
[0099]
[0100] Preparation:
[0101] Valsartan pellets: Pass valsartan, microcrystalline cellulose, and crospovidone through an 80-mesh sieve, mix well, make a soft material with 3% PVP-K30 aqueous solution, and put the soft material in an extrusion-spheronizer Prepare drug-containing micropills, pass the drug-containing micropills through a 16-40 mesh sieve, place in a fluidized bed and coat with Opadry until the weight of the micropills increases by 1.5-3%, dry until the water content is less than 1%, pass through 16 -40 mesh sieves, get valsartan pellets, standby;
[0102] Levoamlodipine pellets: Weigh the prescribed amount of Levoamlodipine and polyvinyl alcohol and disperse them in water, stir until the solution becomes a uniform suspension; put the blank pill core in the fluidized bed, spray the suspension on the drug , after the liquid spraying is completed, spray ...
Embodiment 3
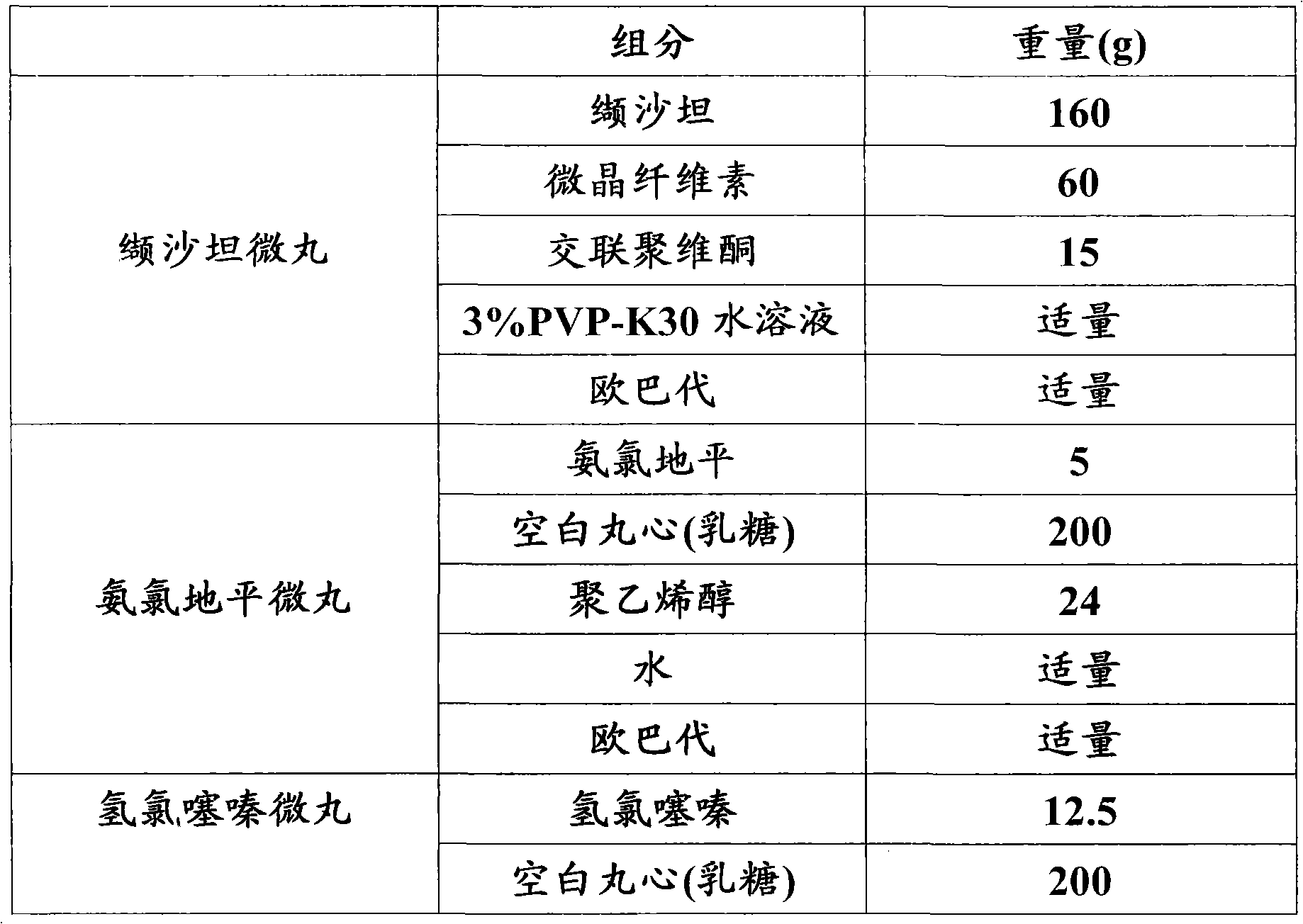
[0106] Embodiment 3: Valsartan amlodipine hydrochlorothiazide pellet capsules (160 / 5 / 12.5mg)
[0107]
[0108]
[0109] Preparation:
[0110] Valsartan pellets: pass valsartan, microcrystalline cellulose, and crospovidone through an 80-mesh sieve, mix well, use 3% PVP-K30 aqueous solution to make a soft material, pass through a 18-mesh sieve to granulate, and release immediately Roll into a coating pan to prepare drug-containing pellets, pass the drug-containing pellets through a 16-40 mesh sieve, dry in a fluidized bed, and coat with Opadry until the weight of the pellets increases by 1.5-3%, dry When the water content is less than 1%, pass through a 16-40 mesh sieve to obtain valsartan pellets for subsequent use;
[0111] Amlodipine pellets: Weigh the prescribed amount of amlodipine and polyvinyl alcohol and disperse them in water, stir until the solution becomes a uniform suspension; put the blank pill core in the fluidized bed, spray the suspension to apply the medi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com