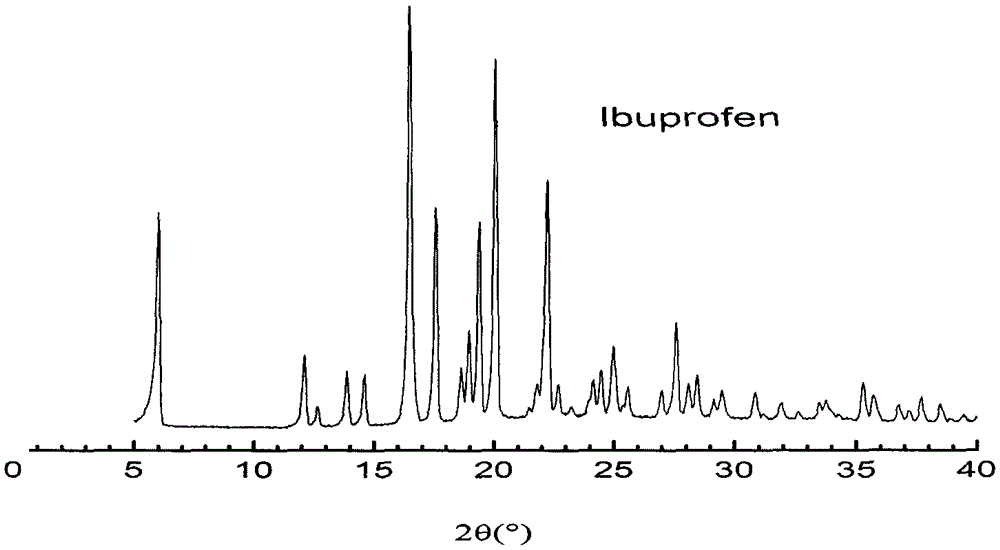
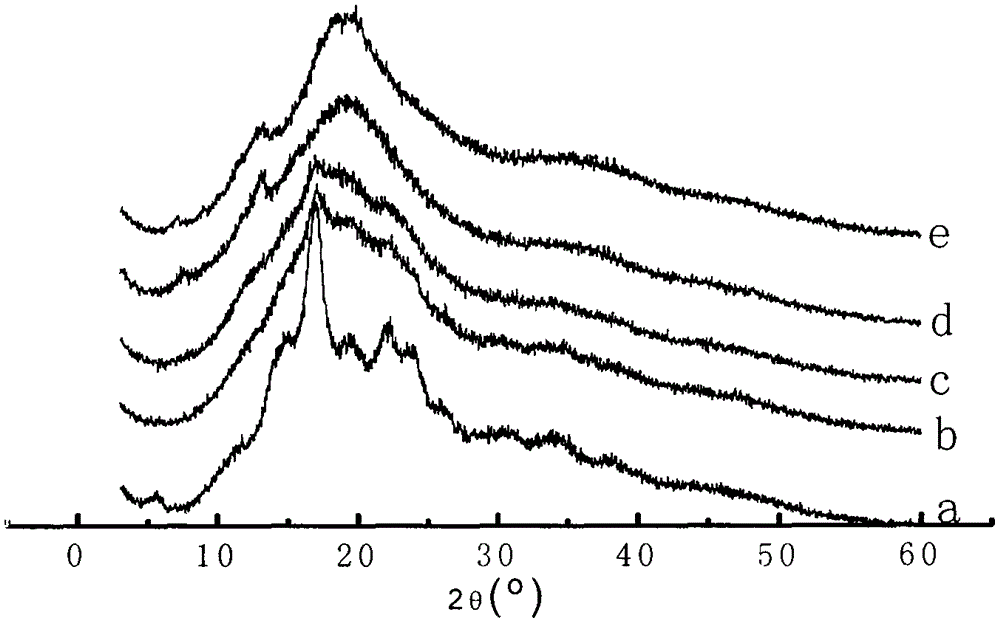
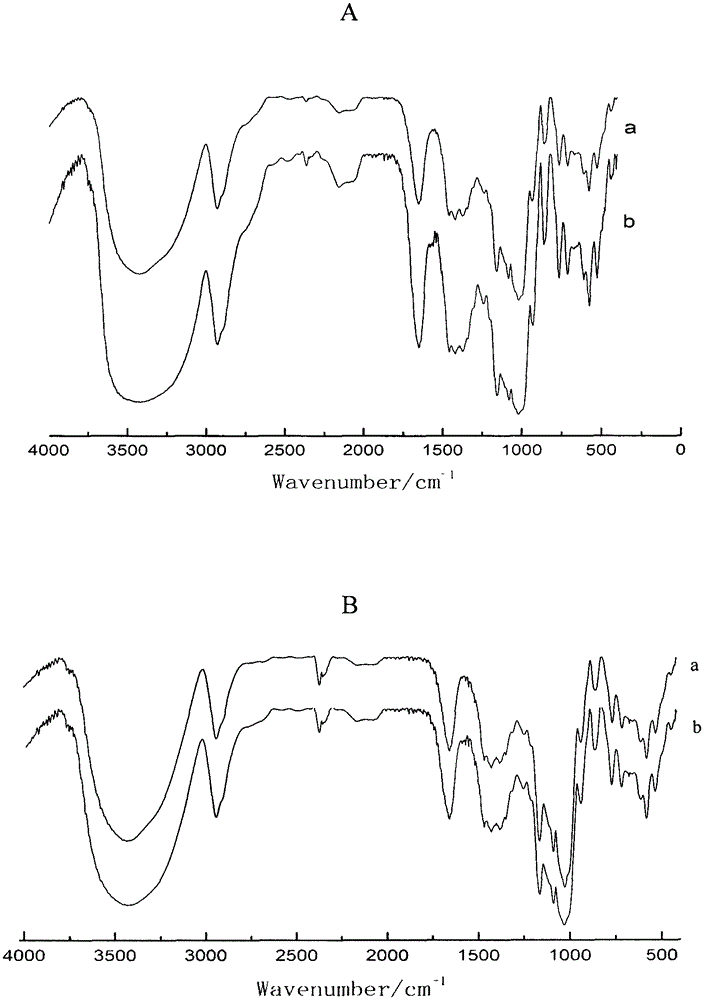
In-situ preparation method for starch-ibuprofen clathrate compound of V-type crystal structure
A technology of in-situ preparation and crystalline structure, applied in the directions of pharmaceutical formulation, drug combination, drug delivery, etc., can solve the problems of high price of β-cyclodextrin, limited application range, poor starch emulsification, etc., and achieves low cost and simplification. The preparation process and the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] a. Accurately weigh a certain amount of ibuprofen (the ratio of ibuprofen and starch is 1:4) and add it to the ethanol solution with a dilution of 70%, stir to make it completely dissolve, then add a certain amount of potato starch to adjust The concentration is 70g / L starch milk, put it into a constant temperature stirrer and keep it at 45°C, and keep stirring;
[0025] b. Add a certain amount of 8mL3mol / L NaOH dropwise into the starch milk at a constant speed of 4mL / min. After the dropwise addition, stir at constant temperature for 60min to make it fully react;
[0026] c. After the reaction is completed, the starch milk is suction-filtered, and the filter cake is dispersed with 60% ethanol solution 20 times the weight of the starch, and the pH is adjusted to 7.0 with 3% (V / V) hydrochloric acid-ethanol solution, and the suction filtration is continued to obtain the filter cake Wash 3 times with 95% ethanol, then wash 1 time with absolute ethanol;
[0027] d. The prod...
Embodiment 2
[0030] a. Accurately weigh a certain amount of ibuprofen (the ratio of ibuprofen and starch is 1:4) and add it to the ethanol solution with a dilution of 50%, stir to make it completely dissolve, then add a certain amount of potato starch to adjust The concentration is 80g / L starch milk, put it into a constant temperature stirrer and keep it at 50°C, and keep stirring;
[0031] b. Add a certain amount of 8mL3mol / L NaOH dropwise to the starch milk at a constant speed of 4mL / min. After the dropwise addition, stir at constant temperature for 40min to make it fully react;
[0032] c. After the reaction is completed, the starch milk is suction-filtered, and the filter cake is dispersed with 60% ethanol solution 20 times the weight of the starch, and the pH is adjusted to 7.0 with 3% (V / V) hydrochloric acid-ethanol solution, and the suction filtration is continued to obtain the filter cake Wash 3 times with 95% ethanol, then wash 1 time with absolute ethanol;
[0033] d. The produc...
Embodiment 3
[0036]a. Accurately weigh a certain amount of ibuprofen (the ratio of ibuprofen and starch is 1:4) and add it to the ethanol solution with a dilution of 50%, stir to make it completely dissolve, then add a certain amount of potato starch to adjust Starch milk with a concentration of 70g / L, put it in a constant temperature stirrer and keep it at 55°C, and keep stirring;
[0037] b. Add 12mL of 3mol / L NaOH dropwise to the starch milk at a constant speed of 4mL / min. After the dropwise addition, stir at constant temperature for 30min to make it fully react;
[0038] c. After the reaction is completed, the starch milk is suction-filtered, and the filter cake is dispersed with 60% ethanol solution 20 times the weight of the starch, and the pH is adjusted to 7.0 with 3% (V / V) hydrochloric acid-ethanol solution, and the suction filtration is continued to obtain the filter cake Wash 3 times with 95% ethanol, then wash 1 time with absolute ethanol;
[0039] d. The product obtained abov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com