Ethyl salicylate glycosides and synthetic method and application thereof
A technology of ethyl salicylate glycosides and compounds, which is applied in the fields of analgesic, anti-inflammatory, and thrombosis-inhibiting drugs, and can solve problems such as many adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
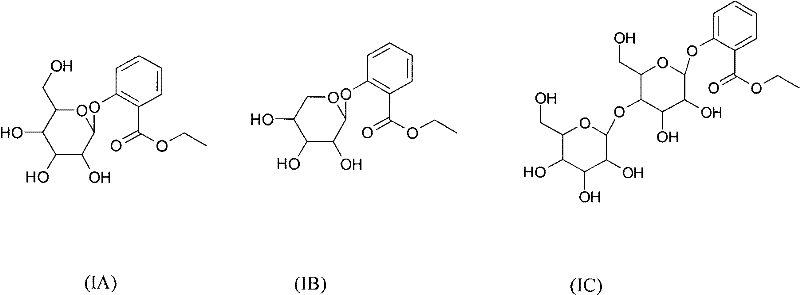
[0139] Example 1 Synthesis of ethyl 2-O-β-D-galactopyranosylbenzoate (1)
[0140] (1) Add 100.0mmol (9.5ml) of acetic anhydride and 1.0mmol (80.0ul) of perchloric acid into a 50ml three-necked flask as a catalyst, stir at room temperature and add 20.0mmol (3.6g) of D-galactose in batches, and control the temperature Lower than 40°C, finish adding within about 30 minutes, continue stirring for about 1 hour, after acetylation is completed, add phosphorus tribromide 10.0mmol (1.1ml) dropwise to the above reaction solution, add about 15 minutes, control the temperature below 20°C , then add 0.2ml of water dropwise, continue stirring for 2-3h, the reaction is completed, pour into ice water, stir, a white solid appears, filter, dissolve in ethyl acetate, wash with water, and dry over anhydrous sodium sulfate to obtain 6.4g of white solid, The yield is about 77.5%.
[0141] (2) A 100ml three-necked flask was placed in an oil bath at 40°C, and 26.0mmol (20.8ml) of 1.25N aqueous sodiu...
Embodiment 2
[0145] Example 2 Synthesis of ethyl 2-O-α-D-mannopyranosylbenzoate (1)
[0146] (1) Add 100.0mmol (9.5ml) of acetic anhydride and 1.0mmol (80.0ul) of perchloric acid into a 50ml three-necked flask as a catalyst, stir at room temperature and add 20.0mmol (3.6g) of D-mannose in batches, and control the temperature Lower than 40°C, finish adding within about 30 minutes, continue stirring for about 1 hour, after acetylation is completed, add phosphorus tribromide 10.0mmol (1.1ml) dropwise to the above reaction solution, add about 15 minutes, control the temperature below 20°C , then add 0.2ml of water dropwise, continue to stir for 2-3h, the reaction is completed, pour into ice water, stir, a white solid appears, filter, ethyl acetate dissolves, wash with water, and dry over anhydrous sodium sulfate to obtain 6.0g of white solid, The yield is about 73.0%.
[0147] (2) A 100ml three-necked flask was placed in an oil bath at 40°C, and 26.0mmol (20.8ml) of 1.25N aqueous sodium hydro...
Embodiment 3
[0151] Example 3 Synthesis of ethyl 2-O-β-D-xylopyranosylbenzoate (3)
[0152] (1) Add 100.0mmol (9.5ml) of acetic anhydride and 1.0mmol (80.0ul) of perchloric acid into a 50ml three-necked flask as a catalyst, stir at room temperature and add 20.0mmol (3.0g) of D-mannose in batches, and control the temperature Lower than 40°C, finish adding within about 30 minutes, continue stirring for about 1 hour, after acetylation is completed, add phosphorus tribromide 10.0mmol (1.1ml) dropwise to the above reaction solution, add about 15 minutes, control the temperature below 20°C , then add 0.2ml of water dropwise, continue to stir for 2-3h, the reaction is completed, pour into ice water, stir, a white solid appears, filter, dissolve in ethyl acetate, wash with water, and dry over anhydrous sodium sulfate to obtain 4.7g of white solid, The yield is about 70.0%.
[0153] (2) Place a 100ml three-necked flask in an oil bath at 40°C, add 26.0mmol (31.2ml) of 1.25N aqueous sodium hydroxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com