Cetirizine and pseudoephedrine sustained-release capsule and preparation method thereof
A technology of sustained-release capsules and cetirizine hydrochloride, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Inhomogeneity, complex processing and other problems, to achieve the effect of improving pharmacokinetic characteristics, increasing bioavailability, and increasing contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
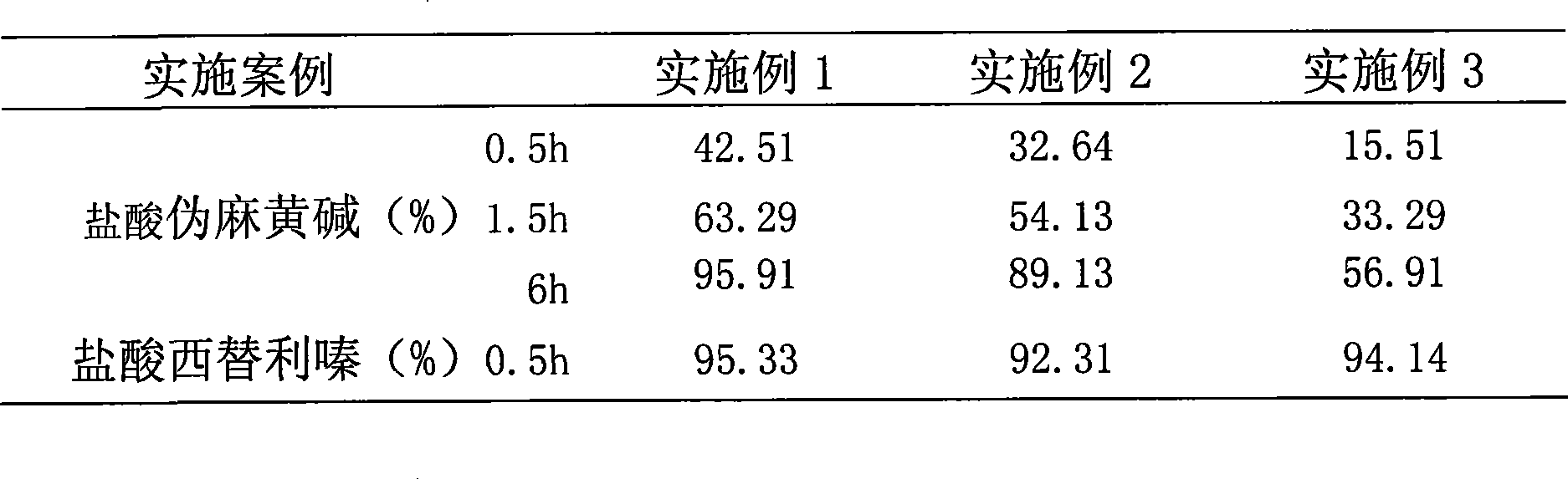
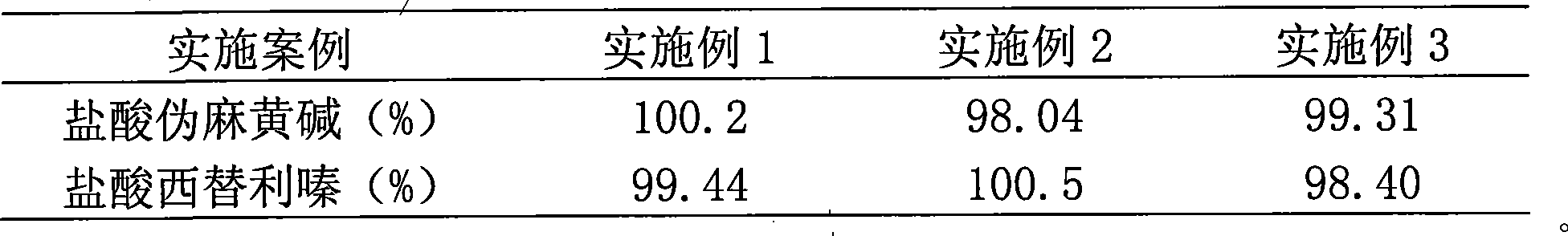
Embodiment 1
[0045] ①Preparation of pseudoephedrine hydrochloride pills with the following weight ratio per 1000 capsules:
[0046] Pseudoephedrine Hydrochloride 120g
[0047] Dextrin 25g
[0048] Blank Ball Heart 30g
[0049] water 50g
[0050] Fully mix pseudoephedrine hydrochloride and dextrin evenly, then put the blank ball core into the multifunctional fluidized bed, and under the state of keeping the multifunctional fluidized bed rotating, gradually add the uniformly mixed materials under the wetting of water Into the multifunctional fluidized bed, wrapped on the blank core, and prepared into drug-containing pellets of pseudoephedrine hydrochloride.
[0051] Contain the following weight proportion system pseudoephedrine hydrochloride pellet coating by every 1000:
[0052] Pseudoephedrine Hydrochloride Pills 175g
[0053] Surelease (solid content 25%) 35g
[0054] water 23g
[0055] The drug-containing pellets were dried in an oven at 60°C. After drying, they were placed in a m...
Embodiment 2
[0070] ①Preparation of pseudoephedrine hydrochloride pills with the following weight ratio per 1000 capsules:
[0071] Pseudoephedrine Hydrochloride 120g
[0072] Dextrin 25g
[0073] Blank Ball Heart 30g
[0074] water 50g
[0075] Fully mix pseudoephedrine hydrochloride and dextrin evenly, then put the blank ball core into the multifunctional fluidized bed, and under the state of keeping the multifunctional fluidized bed rotating, gradually add the uniformly mixed materials under the wetting of water Into the multifunctional fluidized bed, wrapped on the blank core, and prepared into drug-containing pellets of pseudoephedrine hydrochloride.
[0076] Contain the following weight proportion system pseudoephedrine hydrochloride pellet coating by every 1000:
[0077] Pseudoephedrine Hydrochloride Pills 175g
[0078] Su Lisi (solid content 25%) 70g
[0079] water 46g
[0080] The drug-containing pellets were dried in an oven at 60°C. After drying, they were placed in a mul...
Embodiment 3
[0095] ①Preparation of pseudoephedrine hydrochloride pills with the following weight ratio per 1000 capsules:
[0096] Pseudoephedrine Hydrochloride 120g
[0097] Dextrin 25g
[0098] Blank Ball Heart 30g
[0099] water 50g
[0100] Fully mix pseudoephedrine hydrochloride and dextrin evenly, then put the blank ball core into the multifunctional fluidized bed, and under the state of keeping the multifunctional fluidized bed rotating, gradually add the uniformly mixed materials under the wetting of water Into the multifunctional fluidized bed, wrapped on the blank core, and prepared into drug-containing pellets of pseudoephedrine hydrochloride.
[0101] Contain the following weight proportion system pseudoephedrine hydrochloride pellet coating by every 1000:
[0102] Pseudoephedrine Hydrochloride Pills 175g
[0103] Suris (25% solid content) 140g
[0104] water 93g
[0105] The drug-containing pellets were dried in an oven at 60°C. After drying, they were placed in a mult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com