Beta-potastine besilate orally disintegrating tablet and preparation method thereof
A technology for oral disintegrating tablets and benzene sulfonate, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as insufficient bitterness masking effect and residual aftertaste. , to achieve the effect of good disintegration time limit, improved reproducibility, good taste and flavor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
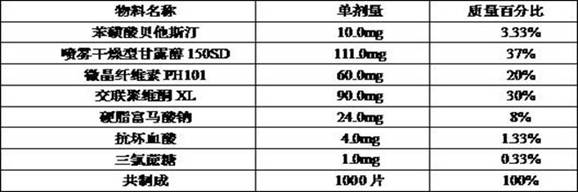
[0078] prescription
[0079]
[0080] Pass the raw and auxiliary materials through a 40-mesh sieve respectively to remove the agglomerates, add the raw and auxiliary materials into the three-dimensional hopper mixer in sequence, set the mixing speed to 20 rpm, and mix for 10 min.
[0081] According to the particle content, the tablet weight should be converted into tablets, and 10.0mm circular shallow arc punching nails were used for tablet compression.
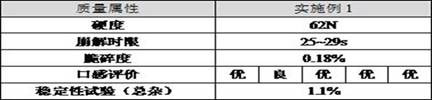
[0082] Experimental results:
[0083]
Embodiment 2
[0085] prescription
[0086]
[0087] Pass the raw and auxiliary materials through a 40-mesh sieve respectively to remove the agglomerates, add the raw and auxiliary materials into the three-dimensional hopper mixer in sequence, set the mixing speed to 20 rpm, and mix for 10 min.
[0088] According to the particle content, the tablet weight should be converted into tablets, and 10.0mm circular shallow arc punching nails were used for tablet compression.
[0089] Experimental results:
[0090]
Embodiment 3
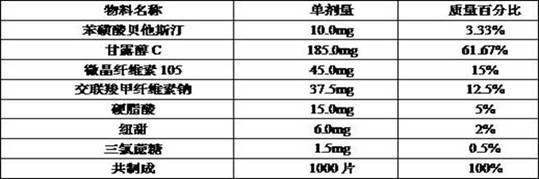
[0092] prescription
[0093]
[0094] Pass the raw and auxiliary materials through a 40-mesh sieve respectively to remove the agglomerates, add the raw and auxiliary materials into the three-dimensional hopper mixer in sequence, set the mixing speed to 20 rpm, and mix for 10 min.
[0095] According to the particle content, the tablet weight should be converted into tablets, and 10.0mm circular shallow arc punching nails were used for tablet compression.
[0096] Experimental results:
[0097]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com