Preparation method of bepotastine besilate tablets
A technology of bepotastine bepotastine tablets and heat-melt production, which is applied in the field of medicine, can solve the problems of instability of bepotastine bepotastine to humidity, etc., and achieve the effects of ensuring product quality, facilitating sample stability, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The prescription components (parts by weight) of the bensulfobestatine tablet of the present application are: 10 parts by weight benzylbepotastine, 8-16 parts of microcrystalline cellulose, 80-92 parts of mannitol, polyethylene 5-15 parts of diol, 0-2 parts of magnesium stearate.
[0019] A typical benzenesulfobestatine tablet has the following components in parts by weight: 10 parts benzenesulfobestatine, 90 parts mannitol, 10 parts polyethylene glycol, 12 parts microcrystalline cellulose, and stearin 1.5 parts of magnesium acid. The preparation method of the benzenesulfobestatine tablet: weigh the benzenesulfobestatine, mannitol and polyethylene glycol in the above proportions, mix them uniformly, and granulate; after the granulation is completed, add microcrystalline cellulose 12 And 1.5 parts of magnesium stearate, mix well, press tablets, and coat to obtain the finished product.
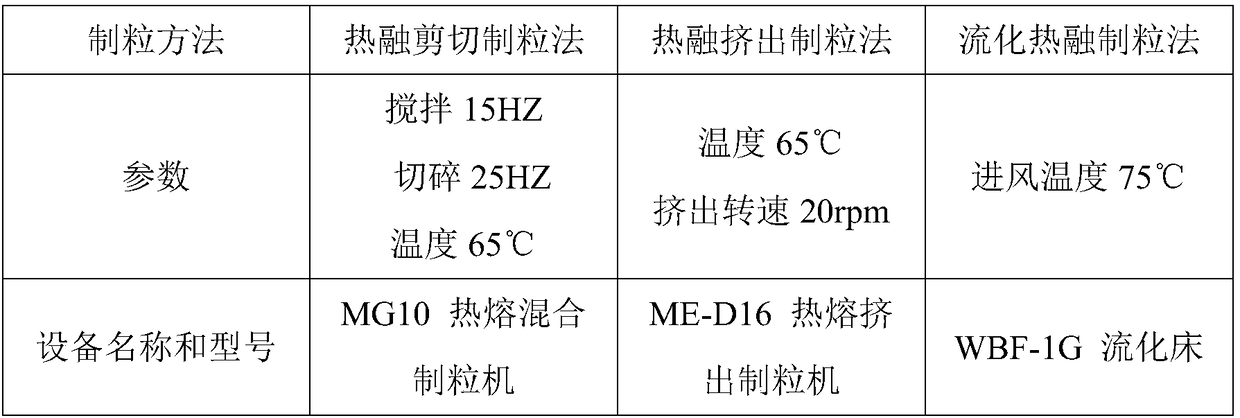
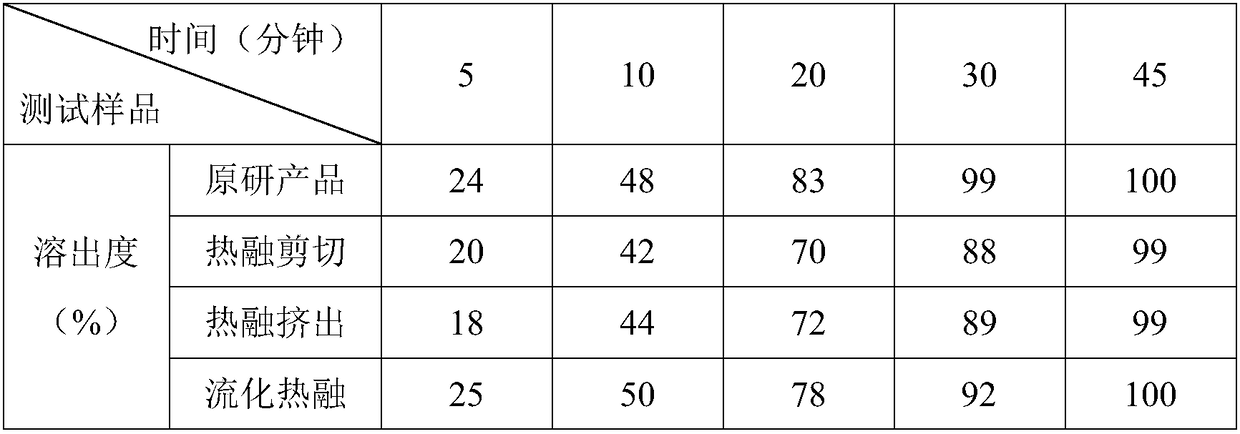
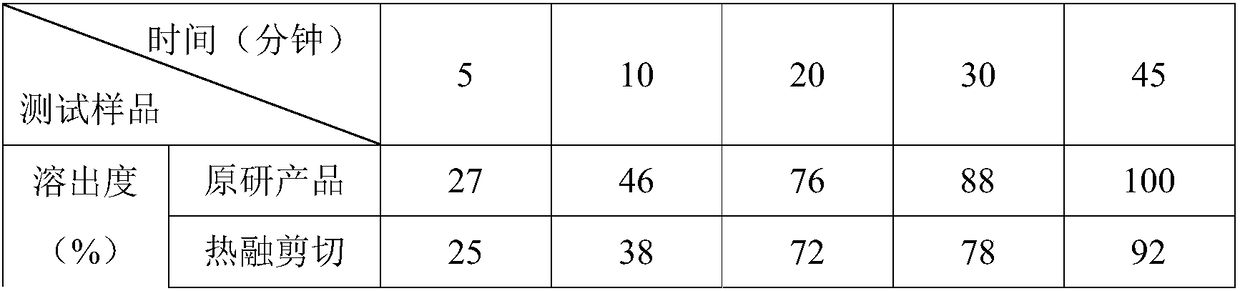
[0020] Among them, three methods of hot melt shear granulation, hot melt extrusion granu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com