Method for separating and measuring Bepotastine Besilate optical isomer impurity
A technology of bepotastine benzenesulfonate and optical isomers, which is applied in the field of separation and determination of optical isomer impurities of drugs, can solve the problems of decreased column efficiency, broadened peak shape, poor durability of chromatographic columns, etc. Good performance, simple mobile phase preparation, and high column efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
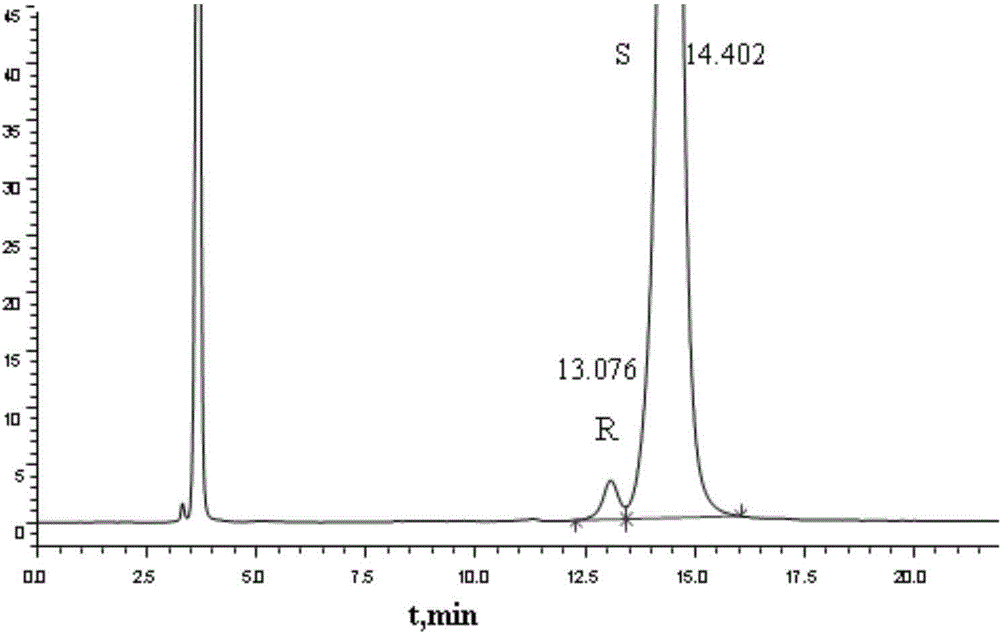
Examples
Embodiment 1
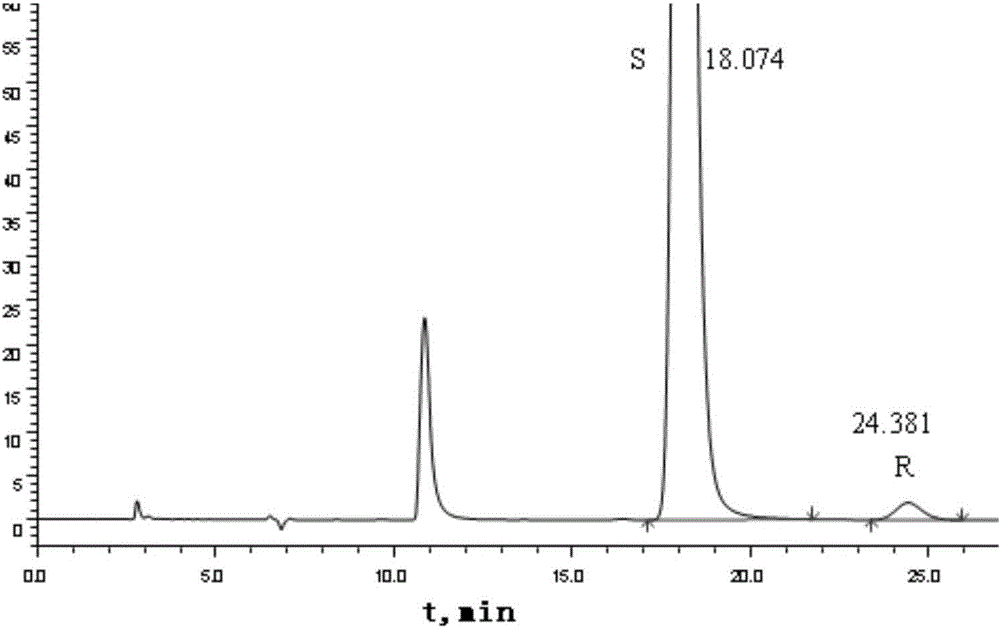
[0039] Example 1 Chiralpak AD-H chromatographic column normal phase HPLC (high performance liquid chromatography) detection
[0040] Chromatographic conditions
[0041] Chromatographic column: ChiralpakAD-H (filler is silica gel coated with amylose-tris(3,5-xylylcarbamate), 4.6×250mm, 5μm)
[0042] Mobile phase: n-hexane-ethanol-ethanolamine (87:13:0.1, V / V / V); n-hexane and ethanol used are chromatographically pure, and ethanolamine is analytically pure
[0043] Detection wavelength: 260nm;
[0044] Injection volume: 20μl; The number of theoretical plates is not less than 2000 based on the peak of bepotastine besilate
[0045] Method: Take appropriate amount of bepotastine bepotastine reference substance and R-isomer reference substance respectively, add appropriate amount of ethanol and ultrasonically dissolve them, and dilute to the mark with n-hexane to make about 1 mg of bepotastine besilate and R-isomer reference substance per 1 ml. For the mixed solution of 50 μg of...
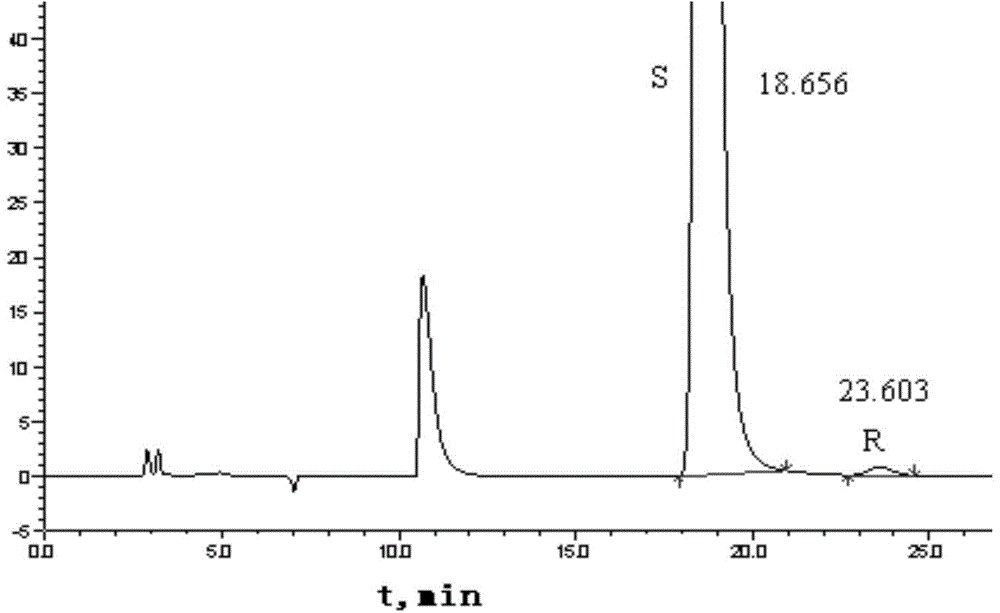
Embodiment 2
[0061] Determination of Optical Isomer Content in Bepotastine Bepotastine Tablets in Embodiment 2
[0062] Carry out normal phase HPLC determination by aforementioned method, instrument and chromatographic condition, concrete operation is as follows:
[0063] Take an appropriate amount of bepotastine bepotastine reference substance and R-isomer reference substance respectively, add appropriate amount of ethanol and ultrasonically dissolve them, and dilute to the mark with n-hexane to make about 1 mg of bepotastine besilate and R-isomer According to the above chromatographic conditions, inject 20 μl of the mixed solution with 5 μg of the body, and record the chromatogram. The sequence of peaks is bepotastine and R-isomer in turn, the number of theoretical plates calculated based on the peak of bepotastine should not be less than 2000, and the degree of separation between bepotastine and adjacent isomers should not be less than 2.0.
[0064] Take three consecutive batches of be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com