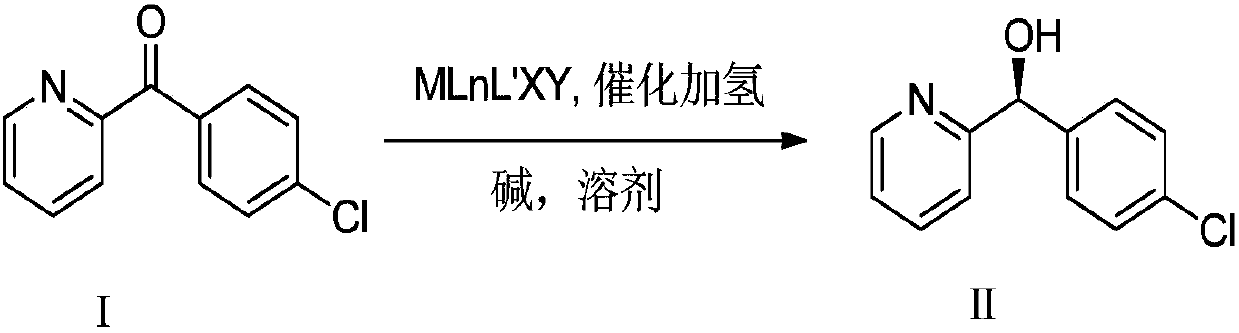
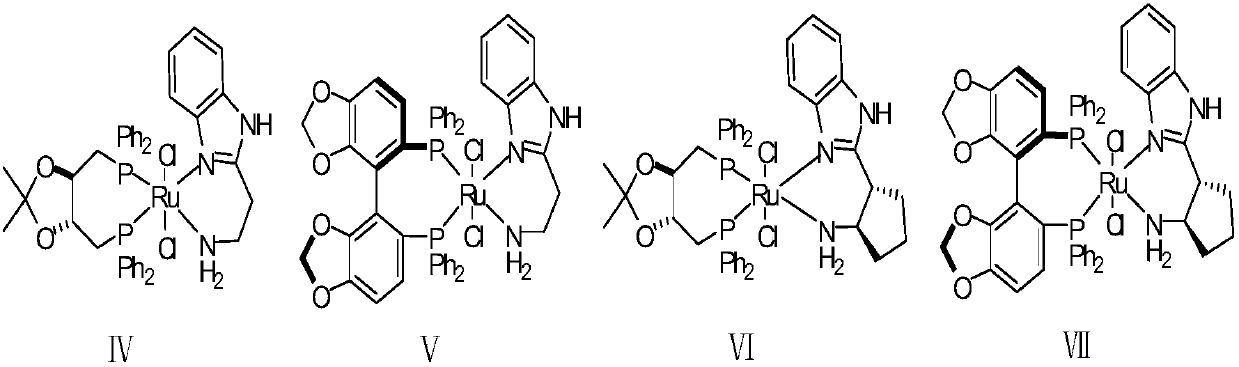
Chiral synthesis method of bepotastine besilate intermediate
A technology of betisine besylate and a synthesis method, applied in the field of chiral synthesis of bepotastine besylate intermediates, can solve the problems of non-industrial production, long steps, low yield and the like, and achieves low production cost, low reaction The effect of stable process and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] In a 5L autoclave, under the condition of connecting argon gas, add 400g of (4-chlorophenyl)(pyridin-2-yl)methanone as a raw material through the feeding port; then add 3L of toluene; Bubble degassing, continuous bubbling for 30min; degassing is complete. Under an argon atmosphere, add 100mg of catalyst (R,R)-DIOPRuCl2(R)-Me-BIMAH through the feeding port, and finally add 6g of potassium tert-butoxide; after the addition, quickly close the feeding port. Replace the argon with hydrogen, slowly introduce hydrogen to 35atm, then close the inflation valve, and close the hydrogen channel; finally stir and keep the reaction at 35°C; the pressure will drop after starting to stir. Observe the pressure change. After 4 hours, the pressure does not change any more. The sample is sent for GC analysis. The conversion rate is 99.7%, and the ee value is 98.2%.
Embodiment 2
[0046] In a 5L autoclave, under the condition of connecting argon gas, add 400g (4-chlorophenyl) (pyridin-2-yl) ketone as a raw material reactant through the feeding port; then add 1L isopropanol and 2L toluene; Inject argon for bubbling degassing, and continue bubbling for 30 minutes; the degassing is completed. Under an argon atmosphere, add 100mg of catalyst (R,R)-DIOPRuCl2(R)-Me-BIMAH through the feeding port, and finally add 6g of potassium tert-butoxide; after the addition, quickly close the feeding port. Replace the argon with hydrogen, slowly introduce hydrogen to 35atm, then close the inflation valve, and close the hydrogen channel; finally stir and keep the reaction at 35°C; the pressure will drop after starting to stir. Observe the change of pressure. After 4 hours, the pressure does not change any more. The sample is sent for GC analysis. The conversion rate is 99.7%, and the ee value is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com