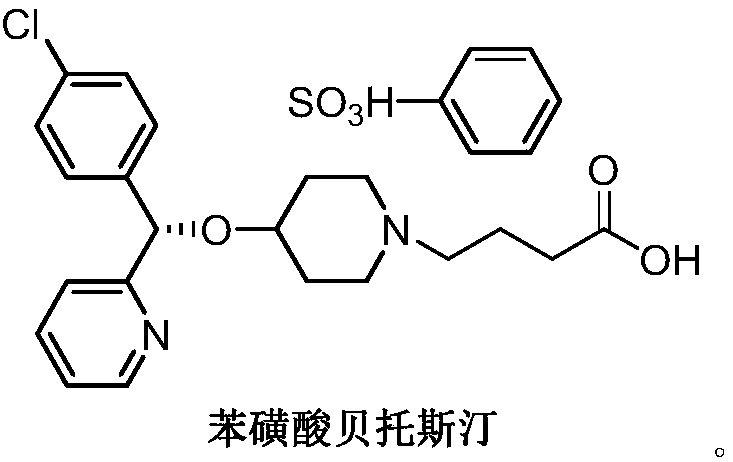
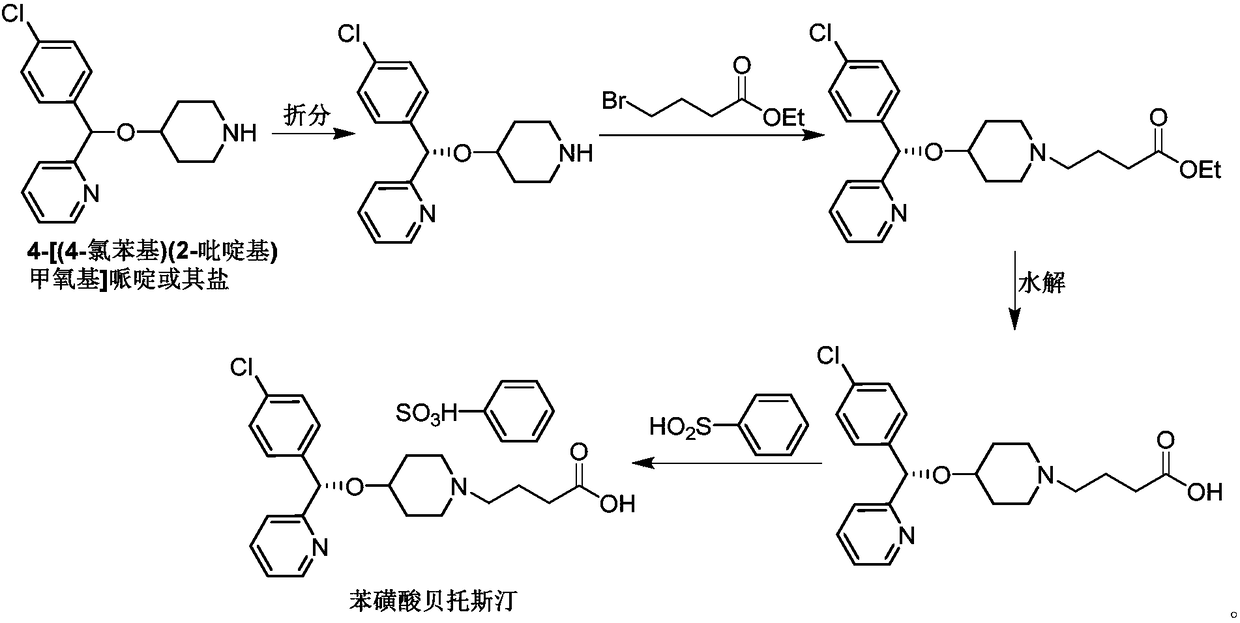
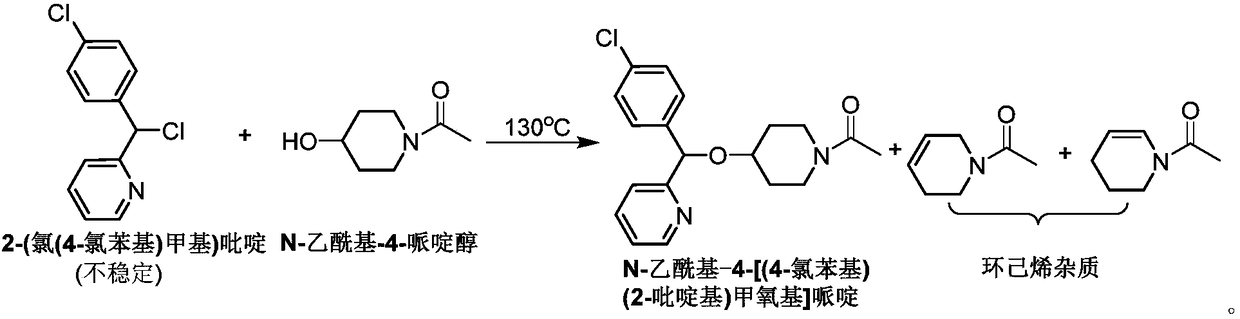
Method for preparing bepotastine besilate key intermediate
A technology of chlorophenyl and formate, which is applied in the field of preparing key intermediates of bepotastine besylate, which can solve the problems of unfavorable large-scale scale-up production and difficulty in convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] 1, preparation formula III (R=Et) compound
[0027] Add compound formula II (X=Cl, R=Et) (200g, 910.5mmol) into a 10L four-necked flask, then add anhydrous toluene (3L), stir well and add NaI (1.36g, 9.1mmol) to the system, then A solution of compound formula I (185 g, 965 mmol) in anhydrous toluene (2 L) was added to the reaction system. After the addition, the system was heated to reflux for 6 hours, and after the reaction, the system was naturally cooled to room temperature. Toluene was distilled off under reduced pressure, and then CH was added to the system 2 Cl 2 (2.5L), add H to the system after stirring 2 O (1L), stirred for 10 minutes, then the system stood still, and the organic phase was separated. CH for aqueous phase 2 Cl 2 (2 x 300 mL) and combine the organic phases. The organic phase was washed with 2% saturated aqueous sodium bicarbonate (300 mL), dried over anhydrous sodium sulfate, filtered, and the organic phase was distilled off under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com