Industrial preparation method of low-cost 2,5-furan dicarboxylic acid
A technology of furandicarboxylic acid and furan formic acid, which is applied in the direction of organic chemistry, can solve the problems of difficult industrial production and high cost, and achieve the effects of reducing reaction cost, simple process and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The invention provides a kind of preparation method of 2,5-furandicarboxylic acid, comprising the following steps:
[0044] 1) by furan formic acid salt, molten salt and catalyst, under the condition of carbon dioxide gas, after carrying out reaction, obtain 2,5-furandicarboxylic acid;
[0045] The melting point of the molten salt is less than or equal to 400°C;
[0046] The catalysts include metal salt catalysts and / or organic base catalysts.
[0047] The present invention is not particularly limited to the furan formate, and the furan formate well known to those skilled in the art can be used. Those skilled in the art can select and adjust according to actual production conditions, quality control and product requirements. The furanic acid salt preferably includes furanic acid potassium salt and / or furanic acid sodium salt, more preferably includes furanic acid potassium salt or furanic acid sodium salt.
[0048] The present invention is not particularly limited to ...
Embodiment 1
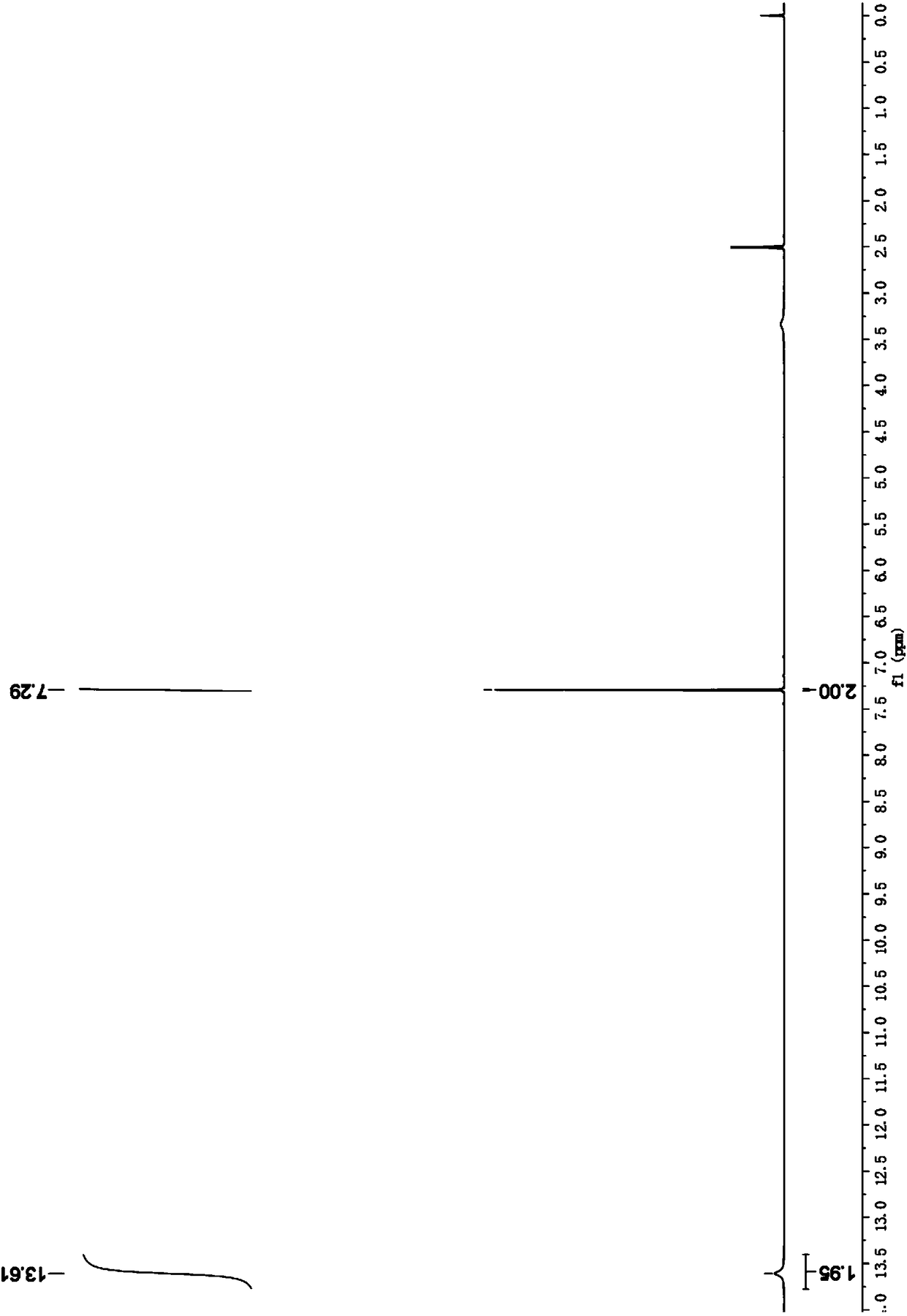
[0077] Into a 100 ml flask, 1.12 g (10 mmol) of furancarboxylic acid, 0.7 g (5 mmol) of potassium carbonate, and 10 ml of water were sequentially added. The obtained clear potassium furanic acid salt solution was evaporated to dryness under reduced pressure to obtain a white solid powder of potassium furanic acid salt. In a 100ml high-temperature and high-pressure reactor, add respectively the mixed molten salt 5.0g of the above-mentioned prepared potassium furoate, anhydrous potassium carbonate 3.45g (25mmol), potassium acetate and sodium acetate (the quality of potassium acetate and sodium acetate The ratio is 1:1). After the addition is complete, replace the air in the reactor with carbon dioxide. Then, under the condition of maintaining a certain air flow, the pressure of carbon dioxide was kept at 0.8 MPa, and the reaction was carried out at 280° C. for 24 hours, and the conversion rate was 95%.
[0078] After the reaction is over, add 100ml of deionized water to the re...
Embodiment 2
[0083] Into a 100 ml flask, 1.12 g (10 mmol) of furancarboxylic acid, 0.7 g (5 mmol) of potassium carbonate, and 10 ml of water were sequentially added. The obtained clear potassium furanic acid salt solution was evaporated to dryness under reduced pressure to obtain a white solid powder of potassium furanic acid salt. In a 100ml high temperature and high pressure reactor, add respectively the mixed molten salt 5.0g (mass ratio of potassium acetate and sodium acetate) of the above-mentioned prepared potassium salt of furanic acid, anhydrous potassium carbonate 3.45g (25mmol), potassium acetate and sodium acetate is 1:1). After the addition is complete, replace the air in the reactor with carbon dioxide. Then, under the condition of maintaining a certain gas flow, the pressure of carbon dioxide was kept at 0.8 MPa, and the reaction was carried out at 280° C. for 6 hours, and the conversion rate was 87%.
[0084] After the reaction is over, add 100ml of deionized water to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com