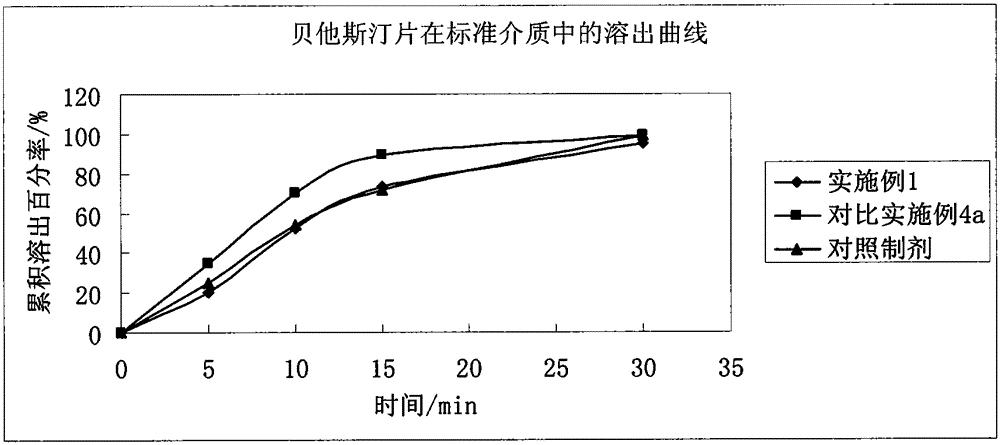
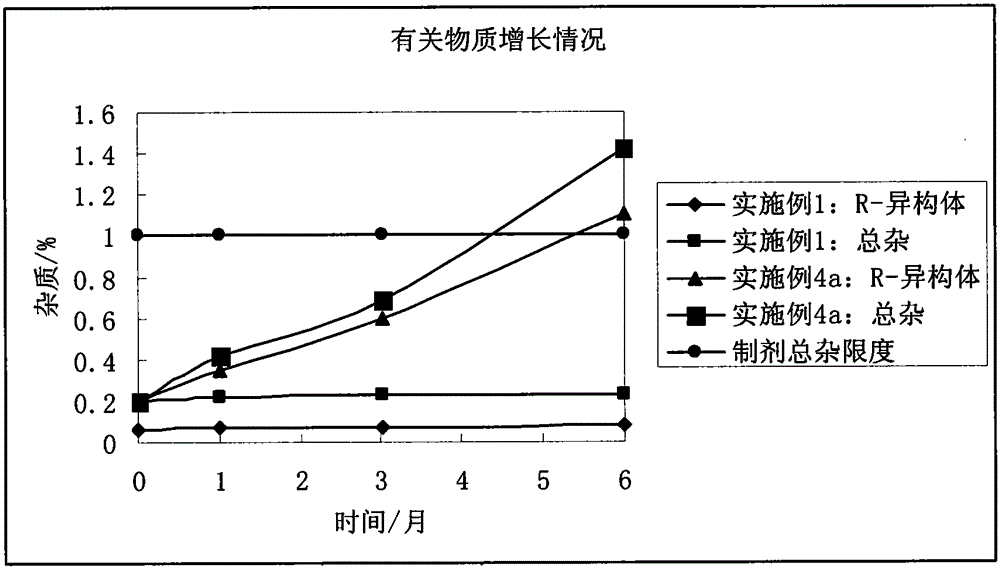
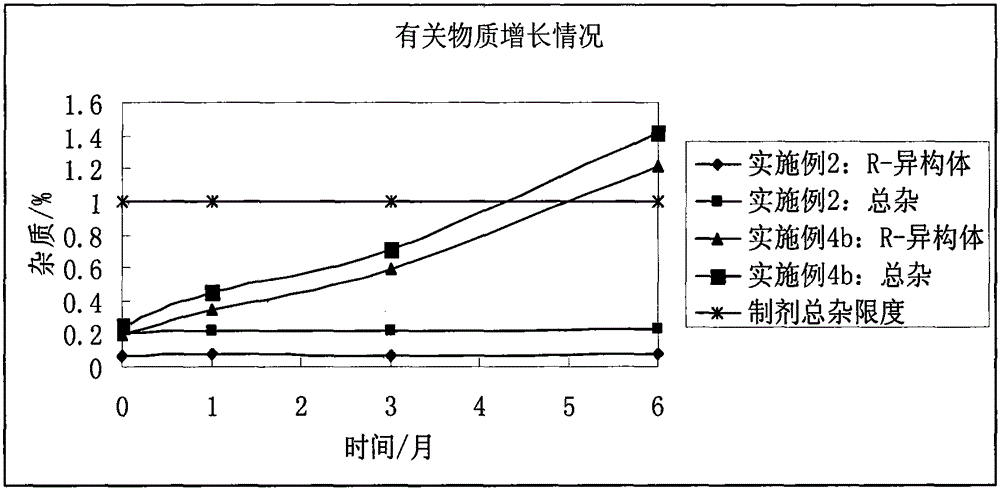
Stable bepotastine besilate tablet and preparation method thereof
A technology of betalastine tablets and stin tablets, which is applied in the directions of medical preparations, pharmaceutical formulations, and pills containing active ingredients, can solve problems such as affecting the stability of preparations, and achieve good stability, good dissolution, and The effect of easy process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: preparation embodiment
[0017]
[0018] Preparation Process:
[0019] (1) Mix 73g of lactose, 28g of microcrystalline cellulose and 4g of hypromellose through an 80-mesh sieve, add an appropriate amount of water-based soft material, and granulate with a 24-mesh granulation to obtain blank granule 1;
[0020] (2) Mix the above-mentioned blank granules 1 with 10 g of bulk drug and 2 g of talcum powder evenly to obtain granules for tableting;
[0021] (3) Tablet pressing with tablet machine, weight 120mg, hardness 70-90N, coated with Opadry.
Embodiment 2
[0022] Embodiment 2: preparation embodiment
[0023]
[0024] Preparation Process:
[0025] (1) Mix 85g mannitol, 41g microcrystalline cellulose, and 5g croscarmellose sodium through an 80-mesh sieve, add an aqueous solution containing 3g hydroxypropyl cellulose to make a soft material, and granulate at 20 mesh to obtain a blank particle 1;
[0026] (2) Mix the above-mentioned blank granules 1 with 11g of bulk drug and 2g of magnesium stearate to obtain granules for tableting;
[0027] (3) Tablet pressing with tablet machine, weight 150mg, hardness 60-80N, coated with Opadry.
Embodiment 3
[0028] Embodiment 3: preparation embodiment
[0029]
[0030]
[0031] Preparation Process:
[0032] (1) Mix 70g of mannitol, 40g of microcrystalline cellulose, 10g of croscarmellose sodium and 5g of croscarmellose sodium through an 80-mesh sieve, add water to make a soft material, and granulate with a 24-mesh to obtain a blank particle 1;
[0033] (2) Mix the above-mentioned blank granule 1 with 9g of bulk drug and 2g of silicon dioxide to obtain granules for tableting;
[0034] (3) Tablet pressing with tablet machine, weight 140mg, hardness 70-90N, coated with Opadry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com