Method for detecting genotoxic impurities in bepotastine besilate
A technology of bepotastine besylate and a detection method, applied in the field of drug analysis, can solve problems such as poor sensitivity and unstable detection of genotoxic impurities, and achieve the effects of high sensitivity, reduction of abnormal chromatographic peaks, and good practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
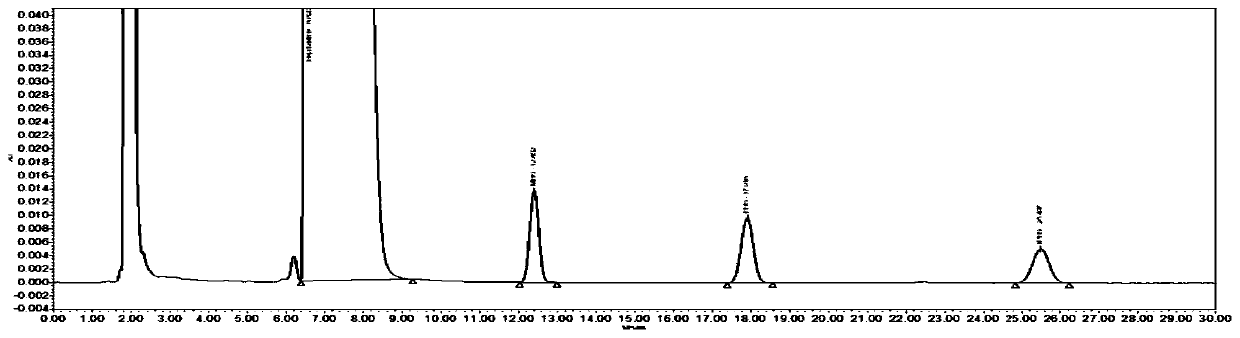
[0045] The detection method of genotoxic impurity in bepotastine besilate comprises the steps:
[0046] (1) Preparation of the test solution: take an appropriate amount of this product powder (approximately equivalent to 40 mg bepotastine besilate), weigh it accurately, put it in a 10ml measuring bottle, add an appropriate amount of acetonitrile, dissolve it by ultrasonication for 2 minutes, and cool to room temperature , dilute to the mark with acetonitrile, shake well, filter, and take the continued filtrate as the test solution.
[0047] (2) Preparation of reference substance solution: take another appropriate amount of reference substances of methyl benzenesulfonate, ethyl benzenesulfonate and isopropyl benzenesulfonate, weigh them accurately, and quantitatively dilute with acetonitrile to make about 4.0 μg in each 1ml solution, as the reference solution.
[0048] (3) High-performance liquid chromatography detection: Precisely measure 20 μl each of the test solution and t...
Embodiment 2
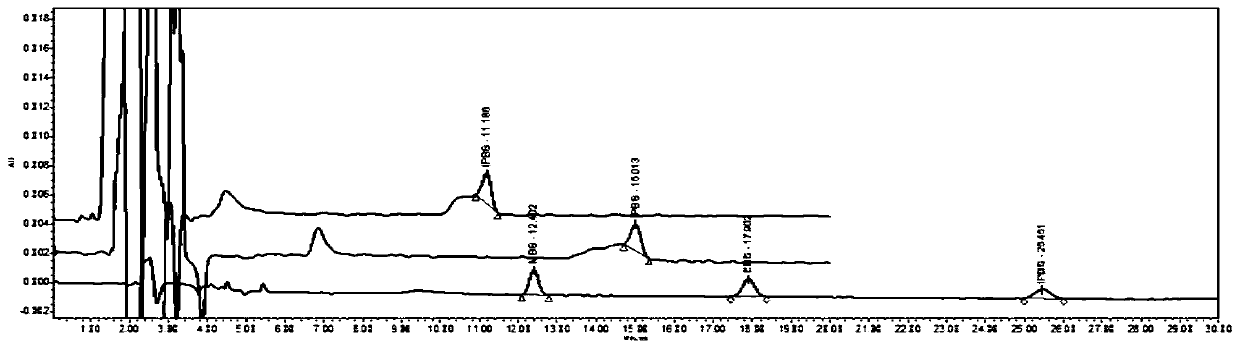
[0085] Investigate the influence of solvent effect on impurity peak shape under different chromatographic columns:
[0086] Diluent: Acetonitrile
[0087] Concentration of isopropyl benzenesulfonate: 1.0μg / ml
[0088] Column:
[0089] Waters Symmetry C18 4.6*250mm, 5μm (carbon load is 19% and specific surface area is 335m2 / g);
[0090] Waters Spherisorb ODS2 C18 4.6*250mm, 5μm (carbon load is 11.5% and specific surface area is 200m 2 / g);
[0091] GL sciences Inertsil ODS3 C18 4.6*250mm, 5μm (carbon loading is 15% and specific surface area is 450m 2 / g);
[0092] from the chromatogram image 3 It shows that the use of GL sciences Inertsil ODS3 C18 can eliminate the abnormal peak shape caused by the solvent effect and make the peak shape of genotoxic impurities meet the requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com