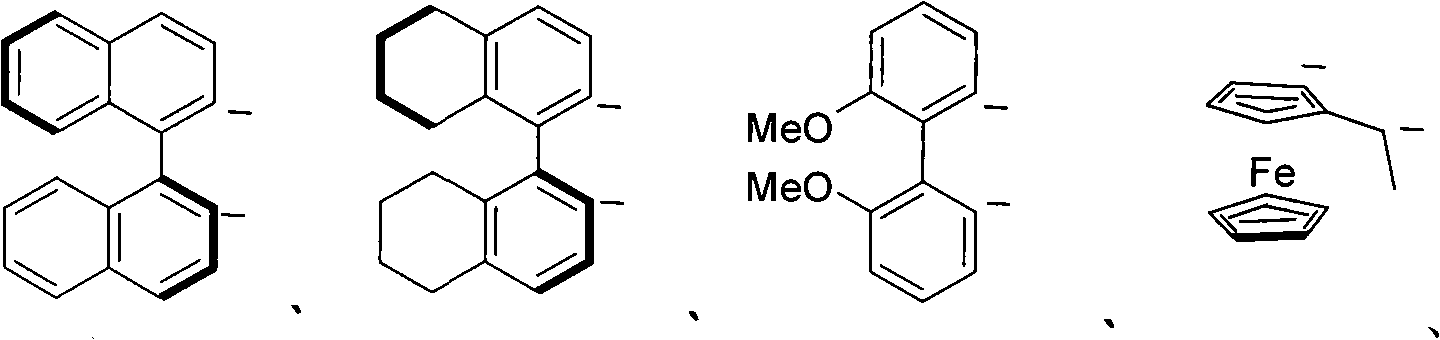
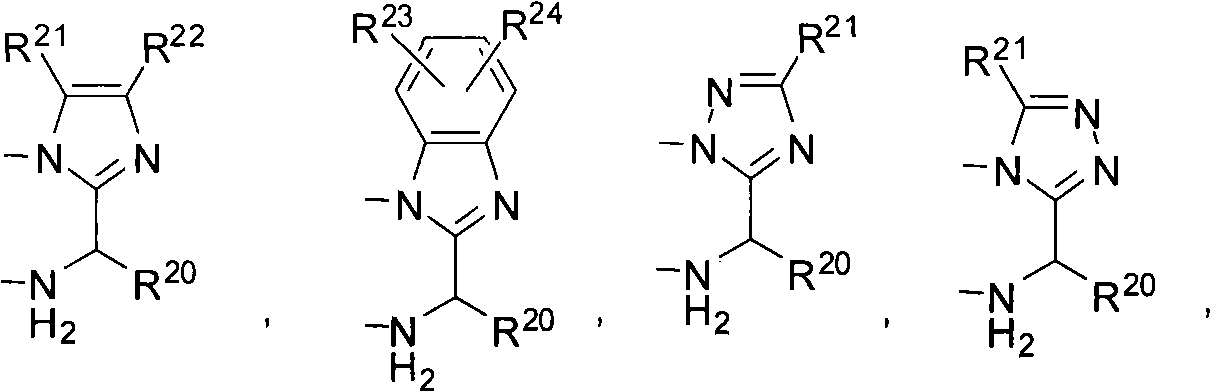
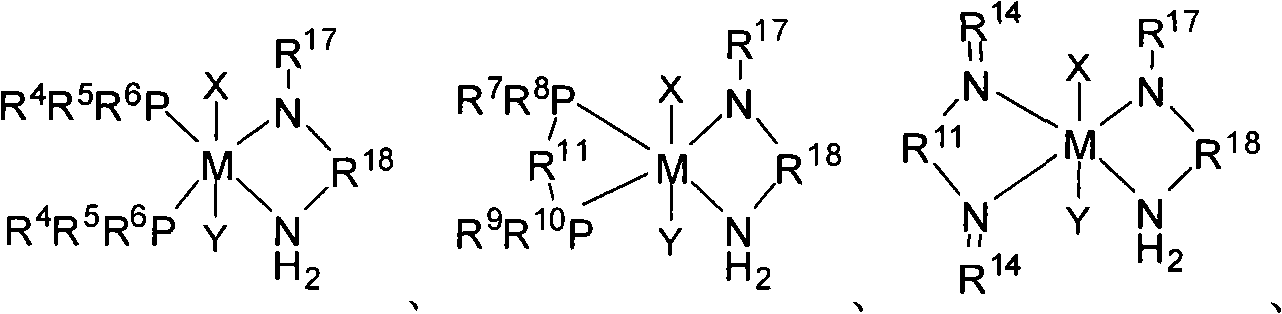
Transient metal complex compound, synthetic method and use thereof
A technology of transition metals and synthesis methods, applied in the field of new transition metal complexes and their synthesis, can solve problems such as difficult universality, and achieve the effect of simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Catalyst preparation
[0033] Preparation method of the present invention can be further embodied as follows with the preparation process of representative compound:
[0034] method one
[0035] [The preparation of catalyst 8 is an example]
Embodiment 1
[0036] Example 1: Catalyst 8
[0037]
[0038] General method (method 1): under the protection of argon, 50mg (0.24mmol) RuCl 3 with 300mg (1.14mmol) PPh 3 Put it into a reaction tube and install a reflux device. Add 12 mL of anhydrous CH 3 OH, heated to reflux for 5 hours. At this time, a brownish-red solid precipitated, filtered under the protection of argon, washed the solid with anhydrous ether, dried the solvent under reduced pressure, and was directly used in the next reaction.
[0039] Under argon protection, put 153 mg (0.16 mmol) of the above powdered solid into a reaction tube, add 2 mL of anhydrous THF, add 140 μL of dry triethylamine and 26 mg (0.16 mmol) of dinitrogen ligand (S)-7, and stir at room temperature After reacting overnight, white insoluble matter precipitated out. Filtrate under Ar atmosphere, drain the filtrate to about 0.5 mL, add 6 mL of anhydrous diethyl ether, a brownish-yellow solid can be precipitated, filter under argon protection, th...
Embodiment 2
[0040] Example 2: Catalyst 9
[0041] Using Method 1, the yield: 76%.
[0042] 31 P NMR (121MHz, CDCl 3 ): δ 44.62 (d, J = 38.2 Hz), 42.80 (d, J = 38.2 Hz), 36.9 (d, J = 11.0 Hz) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com