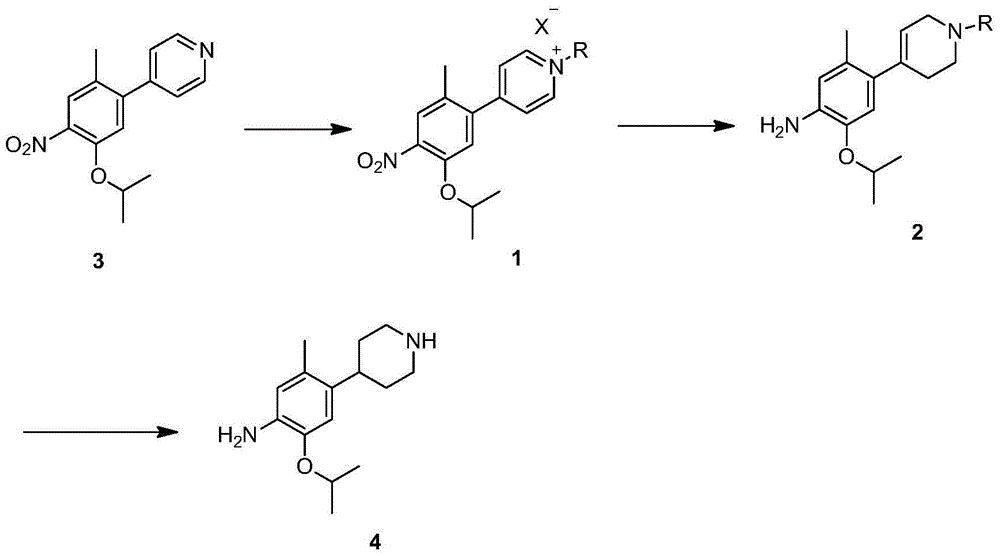
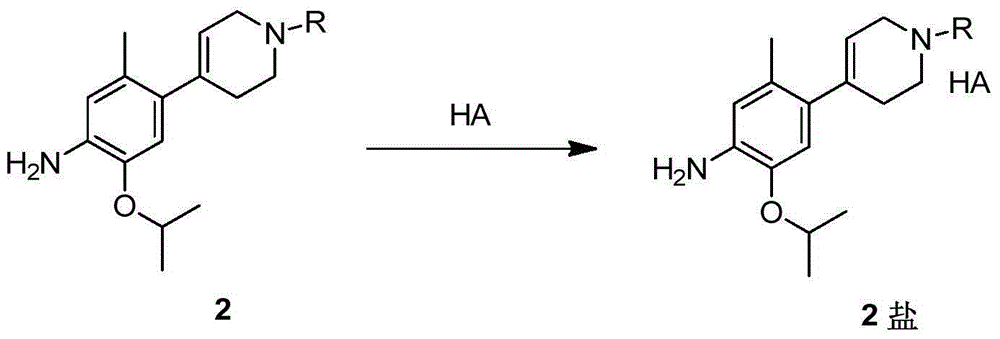
New intermediates for preparing ceritinib and preparation method of intermediate
A technology of ceritinib and intermediates, which is applied in the field of new intermediates and its preparation, can solve problems such as unfavorable drug needs and high product costs, and achieve the effects of reduced material costs, reduced production costs, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
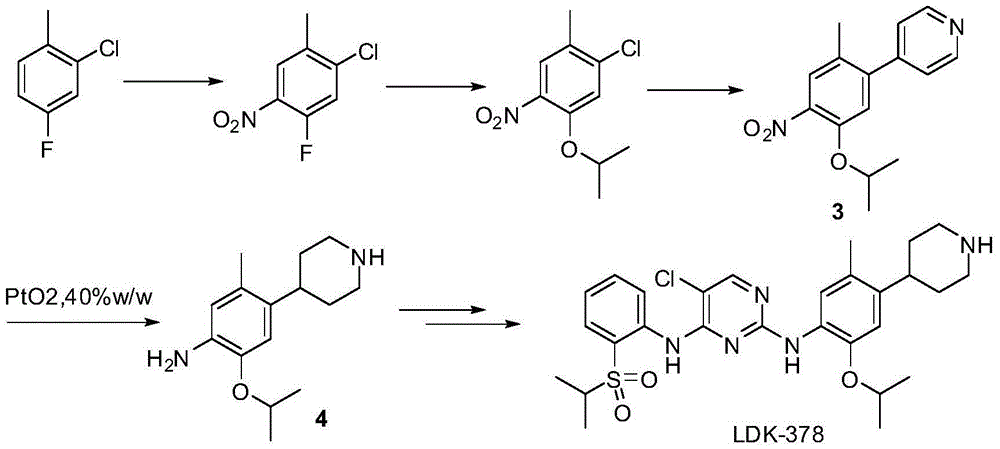
[0023] Embodiment 1. Preparation of 2-chloro-4-fluoro-5-nitro-toluene
[0024]
[0025] Put 135ml of concentrated sulfuric acid into the reaction flask, cool in an ice bath while stirring, and add 43.4g of fuming nitric acid dropwise. Continue to stir for 30 minutes after dropping to form mixed acid. At the same time, 315ml of concentrated sulfuric acid (3.5V) and 90.0g of 2-chloro-4-fluoro-toluene were put into another three-necked flask. Under ice-salt bath cooling, add the above-mentioned mixed acid composed of nitric acid and sulfuric acid into the sulfuric acid solution of 2-chloro-4-fluoro-toluene, and continue the reaction for 1-2 hours. While stirring, the reaction solution was slowly added to crushed ice to quench, and extracted twice with ethyl acetate. The ethyl acetate was combined, washed twice with water and once with saturated brine. The organic phase was concentrated to dryness to give 2-chloro-4-fluoro-5-nitro-toluene as an oil.
[0026] 1 H NMR (CDCl3...
Embodiment 2
[0027] Embodiment 2. Preparation of 2-chloro-4-isopropoxy-5-nitro-toluene
[0028]
[0029]2-Chloro-4-fluoro-5-nitro-toluene was dissolved in 1200ml of isopropanol and added to a 2L three-necked flask. Add 429 g of anhydrous potassium carbonate powder. Warm to reflux with stirring. The reaction was maintained at reflux for ~40 hours. Concentrate to remove most of the isopropanol. 2 L of water was added and extracted twice with ethyl acetate. The ethyl acetate layers were combined and washed with water. Concentration of ethyl acetate gave brown 2-chloro-4-isopropoxy-5-nitro-toluene.
[0030] 1 H NMR (CDCl3): δ7.71 (s, 1H), 7.07 (s, 1H), 4.61 (m, 1H), 2.34 (s, 3H), 1.40 (d, 3.2, 6H).
Embodiment 3
[0031] Example 3. Preparation of 4-(5-isopropoxy-2-methyl-4-nitro-phenyl)pyridine
[0032]
[0033] 78g of 2-chloro-4-isopropoxy-5-nitro-toluene, 42g of 4-pyridineboronic acid, 97g of potassium carbonate, 780mL of dioxane, 390mL of purified water, 7.67g of palladium acetate, and 35.85g of triphenylphosphine g was added to a 2L three-necked flask. Under nitrogen protection, the mixture was stirred and refluxed for 24 hours. Concentrate to remove most of the dioxane. 1.5 L of water was added, and extracted twice with ethyl acetate. The combined ethyl acetate was washed twice with saturated brine, and the organic phase was concentrated to dryness to obtain a brown solid 4-(5-isopropoxy-2-methyl-4-nitro-phenyl)pyridine, the compound 3.
[0034] 1 H NMR(CDCl3):δ8.72(m,2H),7.71(s,1H),7.31(m,2H),6.89(s,1H),4.63(m,1H),2.21(s,3H), 1.38(d,3.0,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com