Synthetic method of 2,5-furan dimethanol and etherified product of 2,5-furan dimethanol
A technology of furan dimethanol and its synthesis method, which is applied in the field of synthesis of 2,5-furan dimethanol and its etherification products, can solve the problems of difficult acquisition of raw materials, high cost of catalysts, etc., and achieve good reusability and reaction system Simple and cost-effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
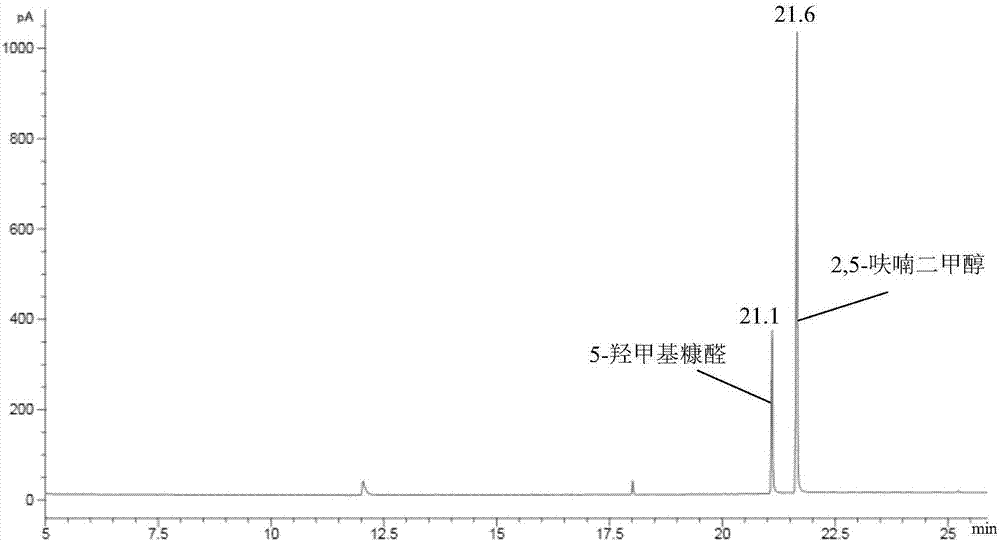
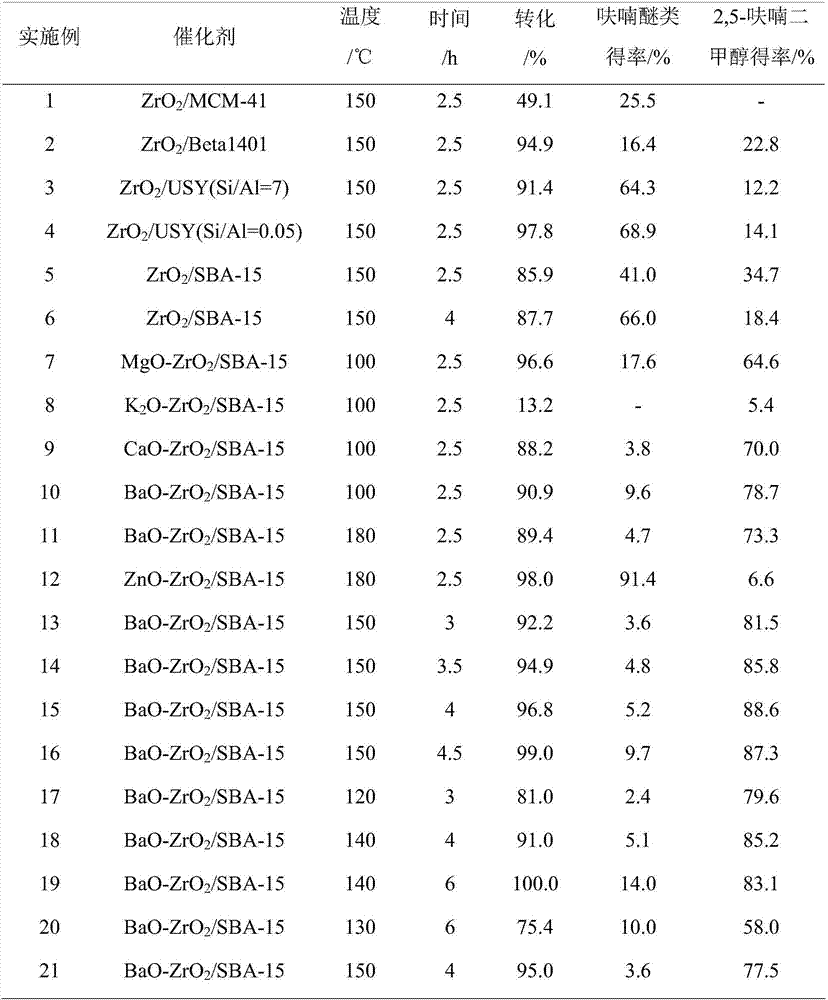
[0025] Add 0.4g 5-hydroxymethylfurfural and 19.6g isopropanol (2wt%) in the autoclave of 50mL, then add 0.2gZrO 2 / MCM-41, ZrO 2 / Beta1401, ZrO 2 / USY, ZrO 2 / SBA-15(ZrO 2 The loading capacity is 40%) as a catalyst, seal the reactor, stir vigorously (500rpm), heat to 150°C and keep for 2.5h, finish the reaction and cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) Qualitative and quantitative tests were carried out, and the test results are listed in Table 1, numbered 1-5.
Embodiment 6
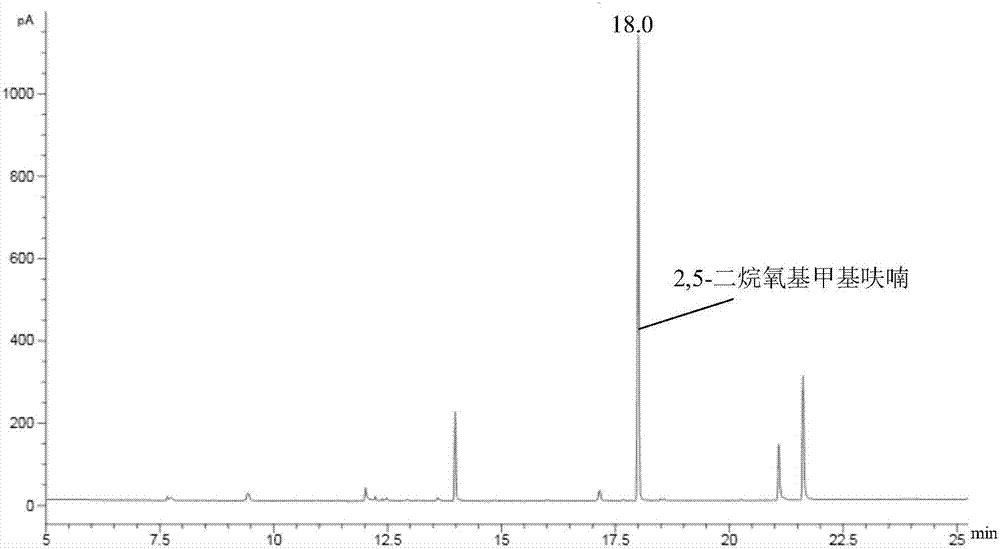
[0027] Add 0.4g 5-hydroxymethylfurfural and 19.6g isopropanol (2wt%) in the autoclave of 50mL, then add 0.2gZrO 2 / SBA-15(ZrO 2 The loading amount is 40wt%) as a catalyst, seal the reactor, vigorously stir (500rpm), heat to 150 ° C and keep for 4h, finish the reaction and cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) to carry out Qualitative and quantitative detection, the detection results are listed in Table 1, the serial number is 6.
Embodiment 7~10
[0029] Add 0.4g 5-hydroxymethylfurfural and 19.6g methanol (2wt%) in the 50mL autoclave, then add 0.2gMgO-ZrO 2 / SBA-15,K 2 O-ZrO 2 / SBA-15, CaO-ZrO 2 / SBA-15, BaO-ZrO 2 / SBA-15 (the load of metal oxide is 40wt%, the mixing ratio of the two metal oxides is 1:1) as a catalyst, seal the reactor, stir vigorously (500rpm), heat to 100°C and keep it for 2.5h, After finishing the reaction, cool down to room temperature and take samples. GC-MS (Shimadzu) and GC (Agilent) are used for qualitative and quantitative detection. The detection results of different catalysts are listed in Table 1, numbered 7-10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com