Method for preparing furan ethers from carbohydrates by one-pot in-situ catalysis
A technology of carbohydrates and in-situ catalysis, which is applied in the field of furan ethers, can solve the problems of long process route, many process unit operations, disadvantages, etc., and achieve the effect of simple process, simple reaction system and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
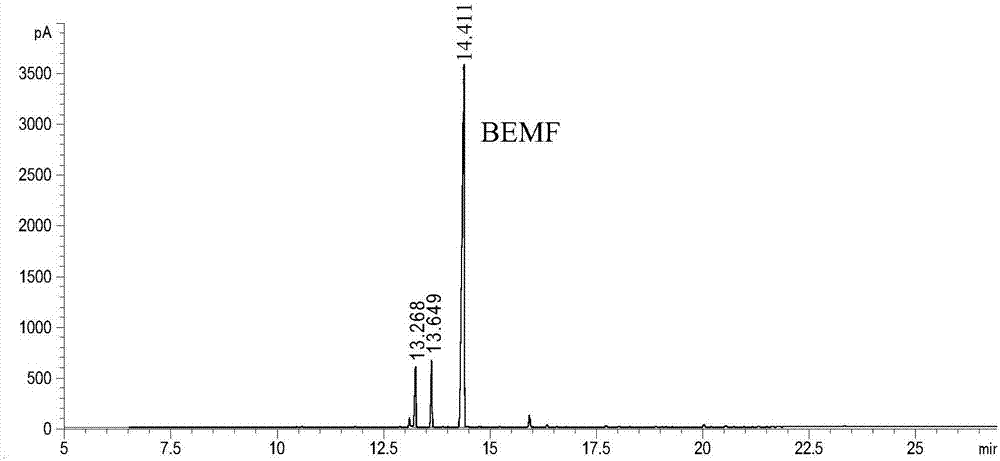
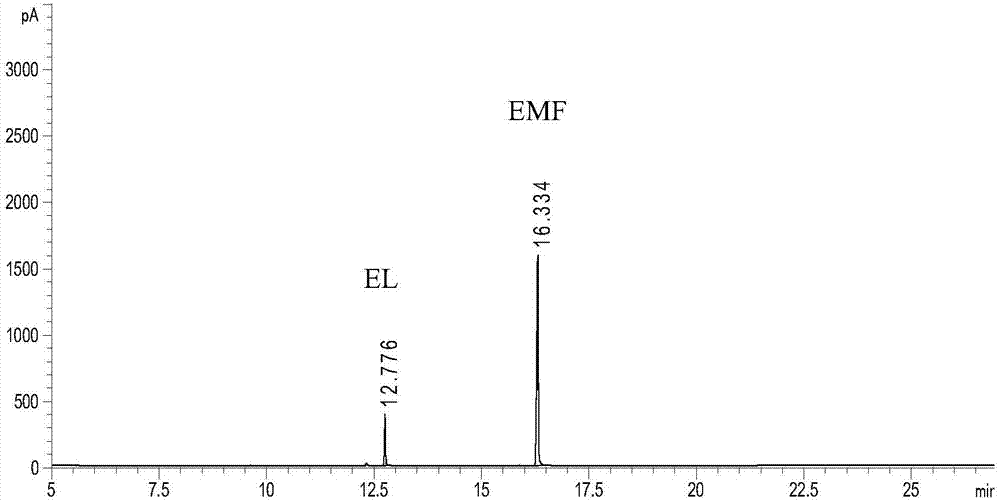
[0021] In the autoclave of 400mL, add 2g fructose and 98g ethanol (2wt%), add the AlCl that is equivalent to the 5mol% of reaction substrate molar weight respectively again 3 ·6H 2 O as a catalyst precursor, seal the reactor, stir vigorously (600rpm), heat to 160°C and keep for 4h, end the reaction, cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) for qualitative and quantitative detection , the detected products include 5-ethoxymethylfurfural (EMF), 2-ethoxymethylfurfuryl alcohol (EFMA), 2,5-diethoxymethylfuran (BEMF), 2-ethoxy Ethylmethyl-5-methylfuran (EMMF), 2-methylfurfural (MF), ethyl levulinate (EL) and 5-ethoxymethylfurfural diethyl acetal (EMFDEA), of which EMF, EMFDEA and BEMF yields reached 16.5%, 16.9% and 13.9%, respectively, and the total product yield reached 56.8%.
Embodiment 2
[0023] Add 2g fructose and 98g ethanol (2wt%) in the autoclave of 400mL, add the ZrOCl that is equivalent to the 5mol% of reaction substrate molar weight respectively again 2 ·8H 2 O as a catalyst precursor, seal the reactor, stir vigorously (600rpm), heat to 200°C and keep for 2h, end the reaction, cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) for qualitative and quantitative detection , the detected EMF and BEMF yields reached 6.3% and 29.6%, respectively, and the total product yield reached 61.4%.
Embodiment 3
[0025] Add 2g fructose and 98g ethanol (2wt%) in the autoclave of 400mL, add the ZrOCl that is equivalent to the 5mol% of reaction substrate molar weight respectively again 2 ·8H 2 O as a catalyst precursor, seal the reactor, stir vigorously (600rpm), heat to 240°C and keep for 2h, end the reaction, cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) for qualitative and quantitative detection , the detected BEMF yield reached 27.7%, and the total product yield reached 43.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com