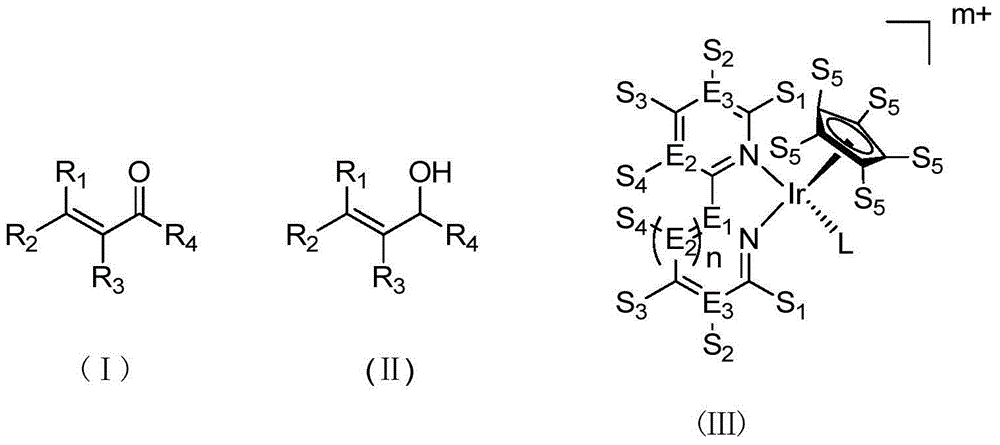
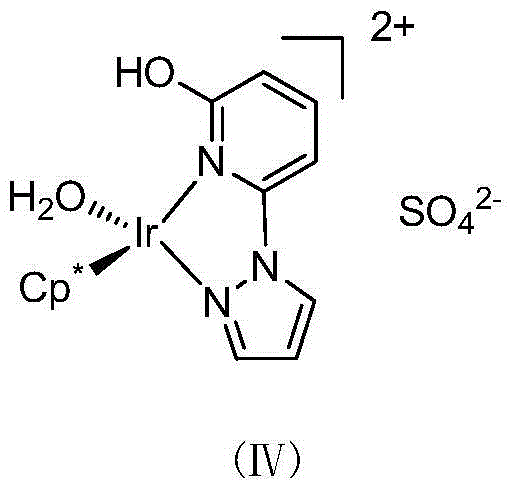
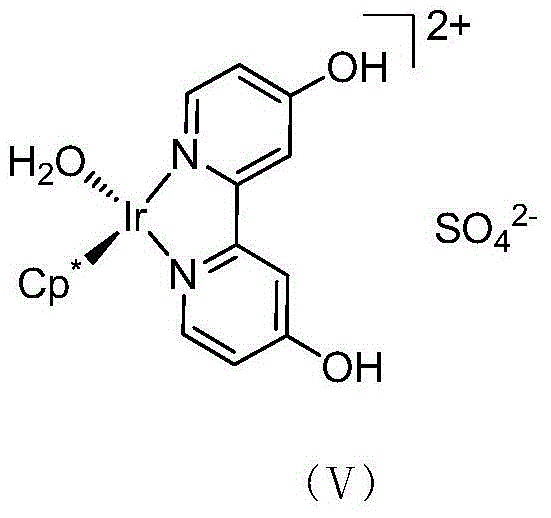
Method for preparing allyl alcohol compounds from alpha,beta-unsaturated aldehyde ketones
An unsaturated compound technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylic acid nitrile, etc., can solve the problem of low catalyst activity, and achieve the effects of high product purity, mild preparation conditions and high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of catalyst stock solution: Add 5.0 μmol of catalyst [Cp*Ir(6-OH-py-pz)(OH 2 )]SO 4 (IV) Add 2 mL of deionized water to prepare a solution with a concentration of 5 μmol / mL. The solution was evacuated / nitrogen replaced three times, then the round bottom flask was frozen in liquid nitrogen under a nitrogen blanket until the solution was completely frozen solid. Then, under vacuum conditions, the round bottom flask was slowly heated in warm water to remove dissolved oxygen and other gases in the aqueous solution. After the solid is completely dissolved, fill it with nitrogen, and perform "liquid nitrogen freezing and degassing-vacuumizing-nitrogen protection thawing" again, repeating three times. Finally, the catalyst solution was stored under nitrogen protection for future use.
[0020] Preparation of β-phenylpropenyl alcohols by transfer hydrogenation of cinnamaldehyde
[0021] To cinnamaldehyde (2.0mmol), according to the molar ratio of cinnamaldehyde a...
Embodiment 2
[0024] The preparation of the catalyst stock solution is the same as that in Example 1. In cinnamaldehyde (2.0mmol), according to the molar ratio of cinnamaldehyde to formic acid / sodium formate, the molar ratio of cinnamaldehyde to formic acid / sodium formate is 1:20, and 2mol / L formic acid / sodium formate aqueous solution (v / v=7 : 1, pH=2.6) 20mL, the solution was evacuated / nitrogen replaced three times, and then the round bottom flask was frozen in liquid nitrogen under nitrogen protection until the solution was completely frozen as a solid. Then, under vacuum conditions, the round bottom flask was slowly heated in warm water to remove dissolved oxygen and other gases in the aqueous solution. After the solid is completely dissolved, fill it with nitrogen, and perform "liquid nitrogen freezing and degassing-vacuumizing-nitrogen protection thawing" again, repeating three times. Then add the prepared [Cp*Ir(4,4'-(OH) 2 -bpy)(OH 2 )]SO 4 (Ⅴ) Catalyst aqueous solution (1 μmol), ...
Embodiment 3
[0027] The preparation of the catalyst stock solution is the same as that in Example 1. In cinnamaldehyde (2.0mmol), according to the molar ratio of cinnamaldehyde to formic acid / sodium formate, the molar ratio of cinnamaldehyde to formic acid / sodium formate is 1:20, and 2mol / L formic acid / sodium formate aqueous solution (v / v=7 : 1, pH=2.6) 20mL, the solution was evacuated / nitrogen replaced three times, and then the round bottom flask was frozen in liquid nitrogen under nitrogen protection until the solution was completely frozen as a solid. Then, under vacuum conditions, the round bottom flask was slowly heated in warm water to remove dissolved oxygen and other gases in the aqueous solution. After the solid is completely dissolved, fill it with nitrogen, and perform "liquid nitrogen freezing and degassing-vacuumizing-nitrogen protection thawing" again, repeating three times. Then add the prepared [Cp*Ir(6,6'-(OH) 2 -bpy)(OH 2 )]SO 4 (VI) Catalyst aqueous solution (1 μmol),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com