Method for preparing 2,5-furandimethanol by transfer hydrogenation of 5-hydroxymethylfurfural
A technology of hydroxymethyl furfural and furan dimethanol, which is applied in 2 fields, can solve the problems of low product selectivity, hydrogen hazardous equipment requirements, uncontrollable selectivity, etc., and achieves high product selectivity, large industrial application value, and preparation process. The effect of green environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 0.26g of 5-hydroxymethylfurfural, 0.1g of catalyst MnO@C-N(700) and 20mL of ethanol into a closed stainless steel reactor, and heat it to 170°C for 25h at a stirring speed of 600rpm. After the reaction, cool to room temperature. The catalyst is centrifuged and the reaction solution is tested. Through gas chromatography analysis, the calculated selectivity of 2,5-furandimethanol is greater than 95%, and the molar yield is 93%.
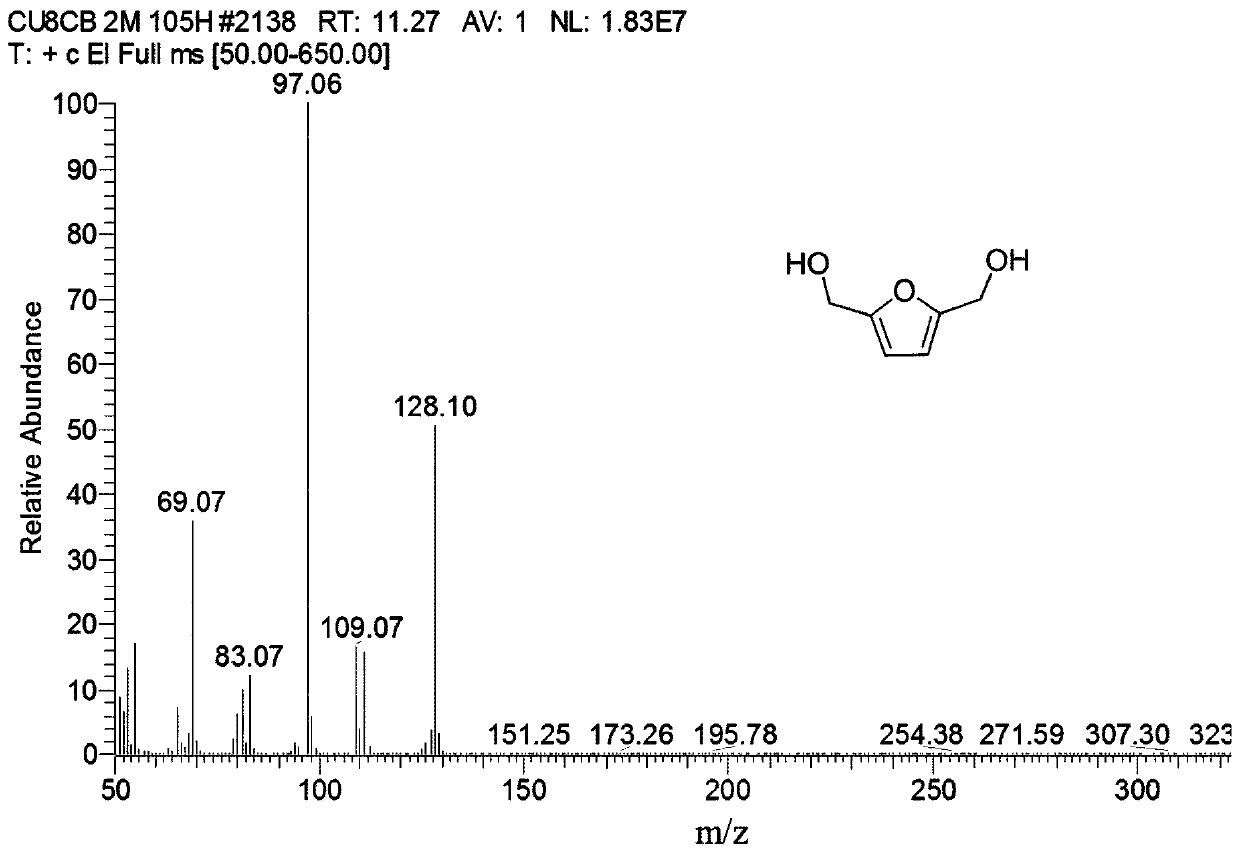
[0030] The GC-MS collection of illustrative plates of the 2,5-furandicarboxylic acid dimethyl that the present embodiment makes is as follows figure 1 shown.
Embodiment 2
[0032] Add 0.26g of 5-hydroxymethylfurfural, 0.1g of catalyst MnO@C-N(700) and 20mL of ethanol into a closed stainless steel reactor, and heat it to 170°C at a stirring speed of 600rpm for 20h. After the reaction, cool to room temperature. The catalyst is centrifuged and the reaction solution is tested. Through gas chromatography analysis, the calculated selectivity of 2,5-furandimethanol is greater than 95%, and the molar yield is 84%. .
Embodiment 3
[0034] Add 0.26g of 5-hydroxymethylfurfural, 0.1g of catalyst MnO@C-N(700) and 20mL of ethanol into a closed stainless steel reactor, and heat it to 170°C at a stirring speed of 600rpm for 10h. After the reaction, cool to room temperature. The catalyst is centrifuged and the reaction solution is tested. Through gas chromatography analysis, the calculated selectivity of 2,5-furandimethanol is greater than 95%, and the molar yield is 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com