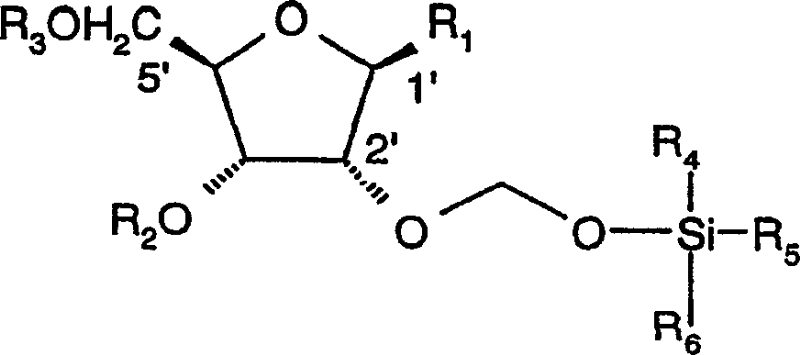
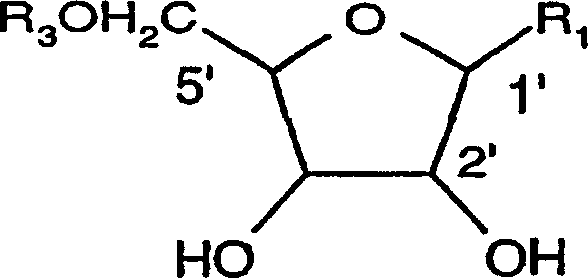
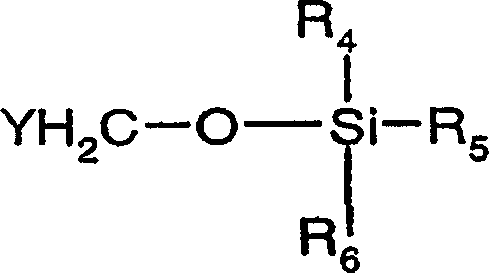
Organic compounds of 2'-o-trisubstituted siloxy methyl ribonucleoside derivatives and their preparation
一种核糖核苷衍生物、衍生物的技术,应用在核酸化学领域,能够解决需要、不适于固相寡核糖核苷酸合成、不可能使用支持体等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0072] 1. Preparation of THEX structural units via an organometallic route
[0073] Scheme 1 depicts a synthetic scheme for the introduction of a THEX protecting group on 5'-O-DMTr uridine followed by phosphitylation.
[0074] Preparation of (1,1,2-trimethyl-propyl)-dimethylsiloxymethyl chloride (THEX-Cl)
[0075] A suspension of 11.1 ml (0.15 mol) ethanethiol and 4.5 g (0.15 mol) paraformaldehyde was treated with 2 drops of NaOMe / MeOH (30%) and stirred at 40° C. for 1 hour. After cooling, add 150ml CH 2 Cl 2 and 22.66 g (0.333 mol) of imidazole. After 10 minutes, 32.66 g (0.167 mol) of (1,1,2-trimethyl-propyl)dimethylsilyl chloride were added dropwise. The resulting suspension was stirred at room temperature for 24 hours and diluted with 300 ml of n-hexane. Add 200ml 2M NaH 2 PO 4 After the solution was stirred (15 min) and the phases were separated, the organic phase was washed with Na 2 SO 4 Dry and evaporate. Dissolve the residue in 100ml CH 2 Cl 2 , with 50m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com