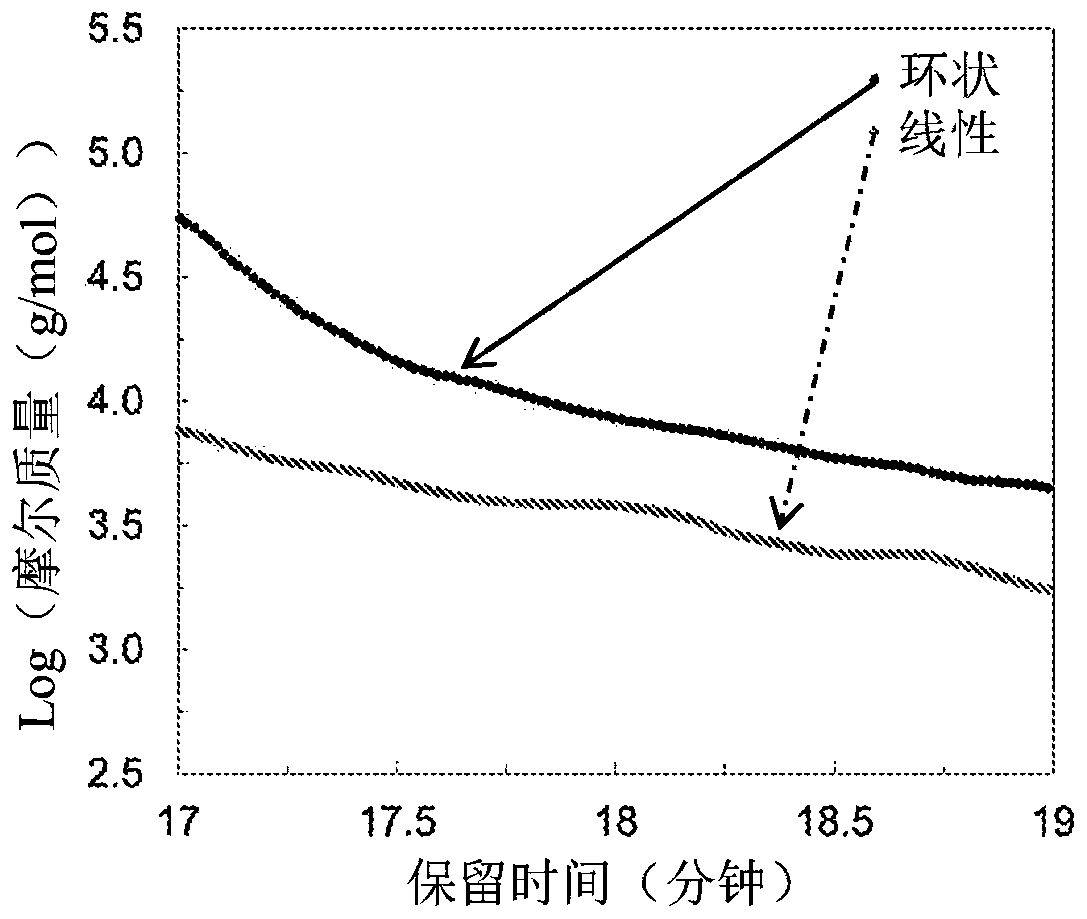
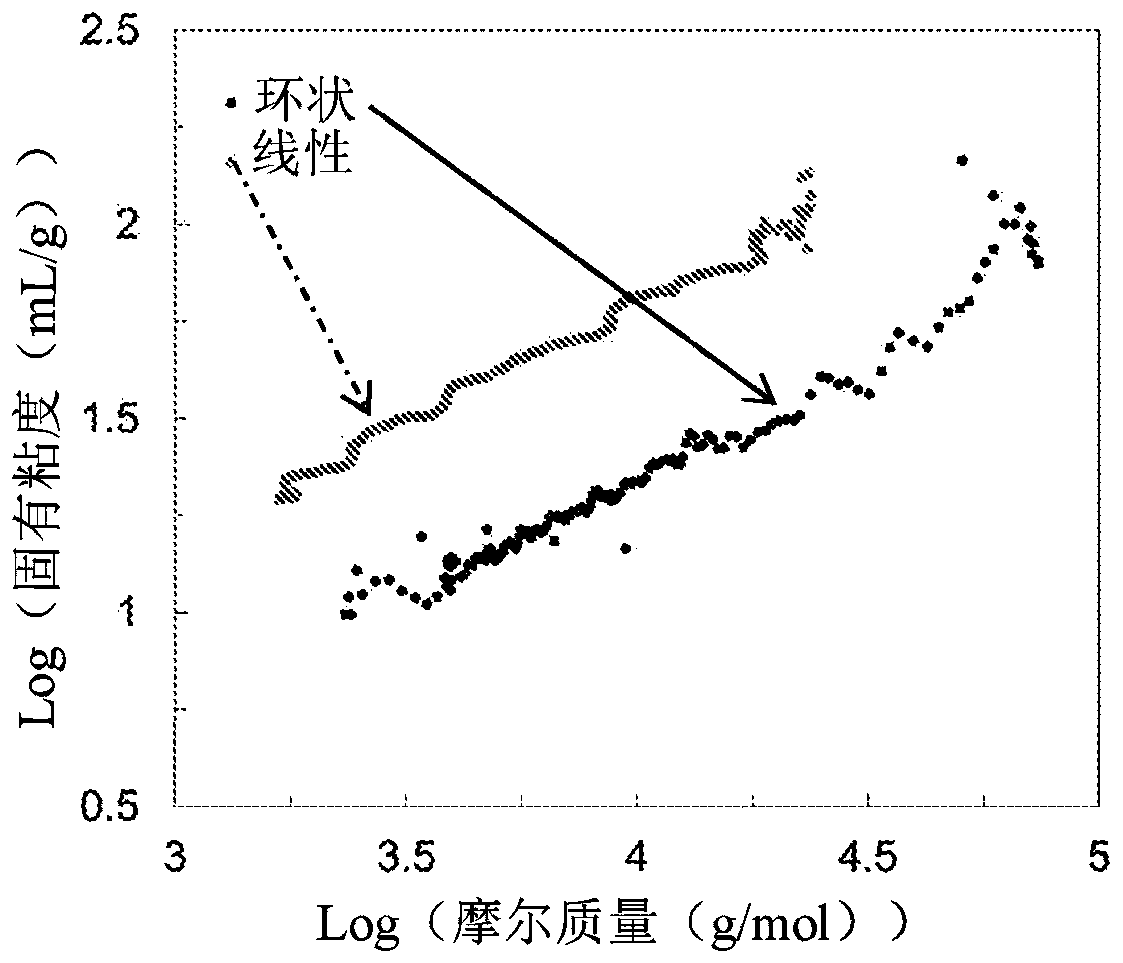
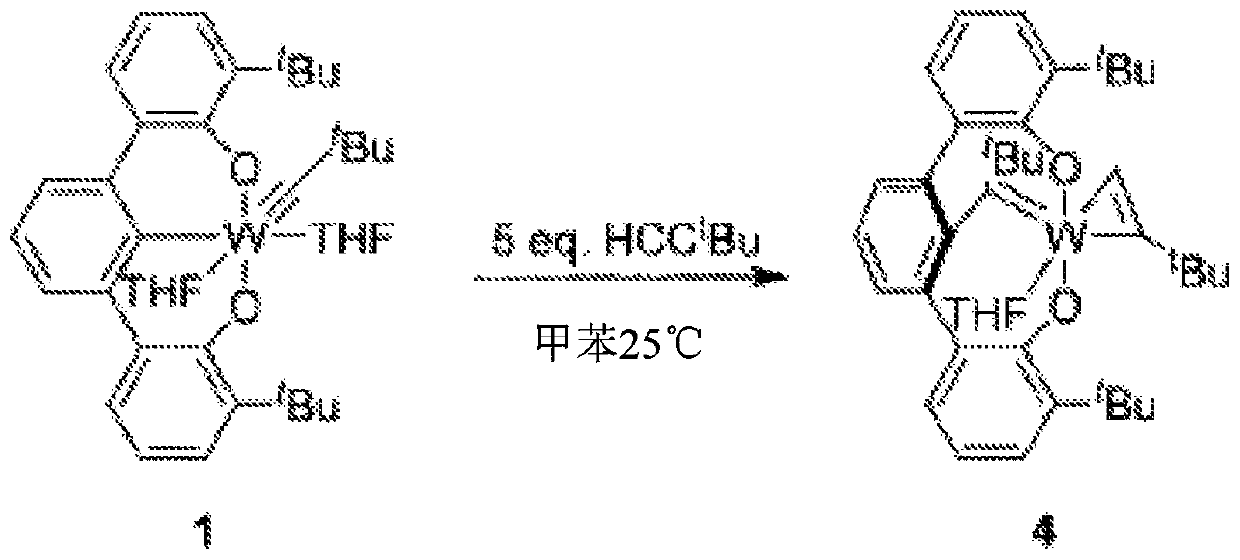
Macrocyclic poly(alkane)s and poly(alkane-co-alkene)s
A technology of cyclic polyolefins and alkanes, applied in the field of cyclic polymers, which can solve the problem of few effective and scalable synthesis strategies for cyclic polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] In the preparation of cyclic poly(olefins), the hydroxyl groups on the monomers can be unprotected or protected as derivatives of the following functional groups: acetyl; benzoyl; benzyl; β-methoxyethoxymethyl ether; methoxymethyl ether; p-methoxybenzyl ether; methylthiomethyl ether; tetrahydropyranyl; tetrahydrofuran; trityl; trimethylsilyl; tert-butyldimethyl tri-isopropylsilyloxymethyl; triisopropylsilyl; methyl ether; ethoxyethyl ether; or protected in any other way. In the preparation of cyclic poly(alkenyl); the amino group on the monomer can be unprotected or protected as a derivative of the following functional groups: carbenzyloxy; p-methoxybenzylcarbonyl; tert-butoxycarbonyl; 9-fluorenylmethoxycarbonyl; benzyl; p-methoxybenzyl; 3; 4-dimethoxybenzyl; or protected in any other way. In the preparation of cyclic poly(alkenyl); the carboxylic acid group on the monomer can be unprotected or protected as a derivative of the following functional groups: methyl ester;...
example 1
[0116] Example 1: Macrocyclic poly(propyne) and polypropylene
[0117] Polymerization of propyne was carried out in propyne-saturated dry THF formed by bubbling propyne gas for three minutes. Polymerization was initiated by injecting tungsten catalyst complex 4 into the reaction flask through the septum. The solution immediately turned orange as the viscosity increased and the temperature increased. Addition of dry and oxygen-free methanol after 15 minutes terminates the polymerization and precipitates polymer which forms a fibrous material after drying overnight. Polymer 1 The H NMR spectrum exhibited a broad peak signal of olefinic protons ranging from 4.6 to 6.5 ppm. The broad signal contained a maximum at 5.9 ppm and was attributable to the trans isomer protons of polypropyne. The methyl protons appear as broad signals centered at 0.89ppm and 1.79ppm, and 13 C{ 1 H} NMR spectrum contains a resonance at 135.1 ppm, indicating sp 2 carbon. These assignments are consist...
example 2
[0125] Example 2: Macrocyclic poly(4-methyl-1-pentene)
[0126] In an inert atmosphere glove box, toluene (2.0 mL) and 4-methyl-1-pentyne (600 μL, 5.0 mmol) were added to a glass vial equipped with a stir bar to obtain a colorless monomer solution. The stock solution of tungsten catalyst complex 4 and the monomer solution were injected once at a ratio of 1700:1 via a micropipette at ambient temperature to initiate polymerization. Polymerization was rapid and exothermic. The color of the solution immediately changed from light yellow (catalyst) to bright orange. After 30 minutes, the reaction solution was added dropwise to a ten-fold excess of stirred degassed methanol, resulting in an orange precipitate. Vacuum filtration under a flow of argon followed by drying under vacuum overnight afforded cyclic poly(4-methyl-1-pentyne) (cPMPy) in 95.5% yield, M n 5.80×10 5 and the dispersion is 3.76. 1 H NMR (CDCl 3 ,300MHz)δ(ppm):6.3-5.5(b,1H,CH=C),2.5-1.1(bm,3H,CH 2 -CH),1.0-0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com