Aminopherase mutant and application thereof in production of L-glufosinate-ammonium
A mutant, transaminase technology, applied in the field of bioengineering, can solve the problems of low process efficiency and conversion rate of only 52%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
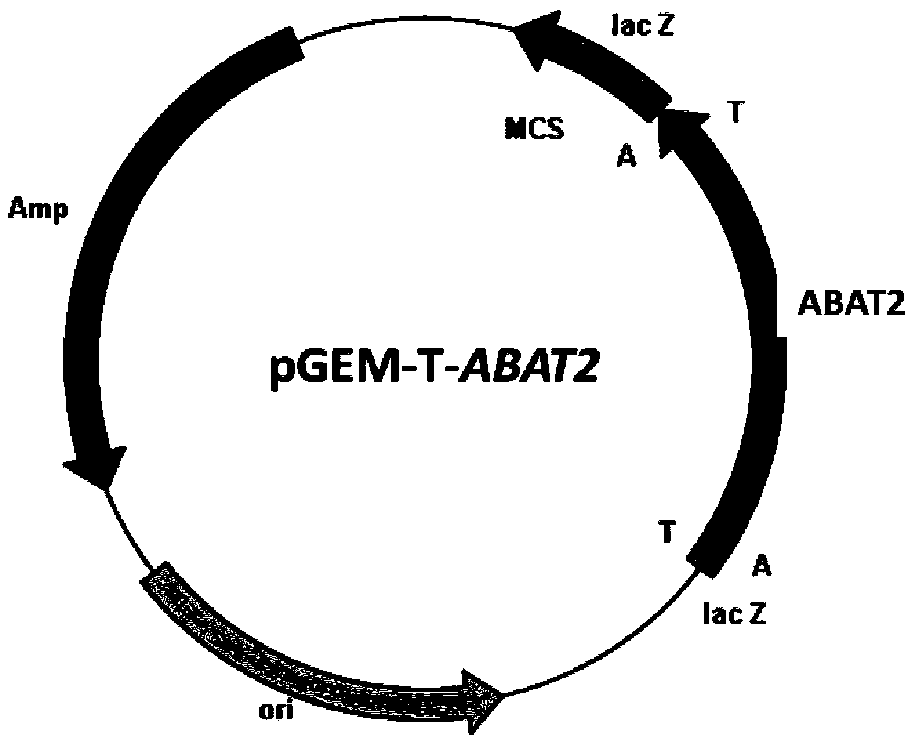
[0038] Example 1: Amplification of transaminase gene ABAT2
[0039] Pseudomonas fluorescens ZJB09-108 is isolated from the soil and stored in the China Center for Type Culture Collection (preservation number CCTCC NO: M2012539, which has been disclosed in the patent application, application number CN 201210593105.3, published (announcement ) No. CN103131649A).
[0040] Based on the transaminase gene sequencing information from Pseudomonas (WP_076423369.1) included in Genbank, a rapid nucleic acid extraction instrument was used to extract the total genomic DNA of Pseudomonas (thalline, and the genomic DNA was used as a template, and primer 1 (5'-ATGAACACCAACAACGCTC-3') and primer 2 (5'-TTAAGCCTGTTTAGCTTC-3') for PCR amplification. PCR reaction system (total volume 50 μL): 10×Pfu DNA Polymerase Buffer 5 μL, 10 mM dNTP mixture (dATP, dCTP, dGTP and dTTP each 2.5 mM) 1 μL, cloning primer 1 and primer 2 each 1 μL at a concentration of 50 μM, genomic DNA 1 μL, Pfu DNA Polymerase 1 ...
Embodiment 2
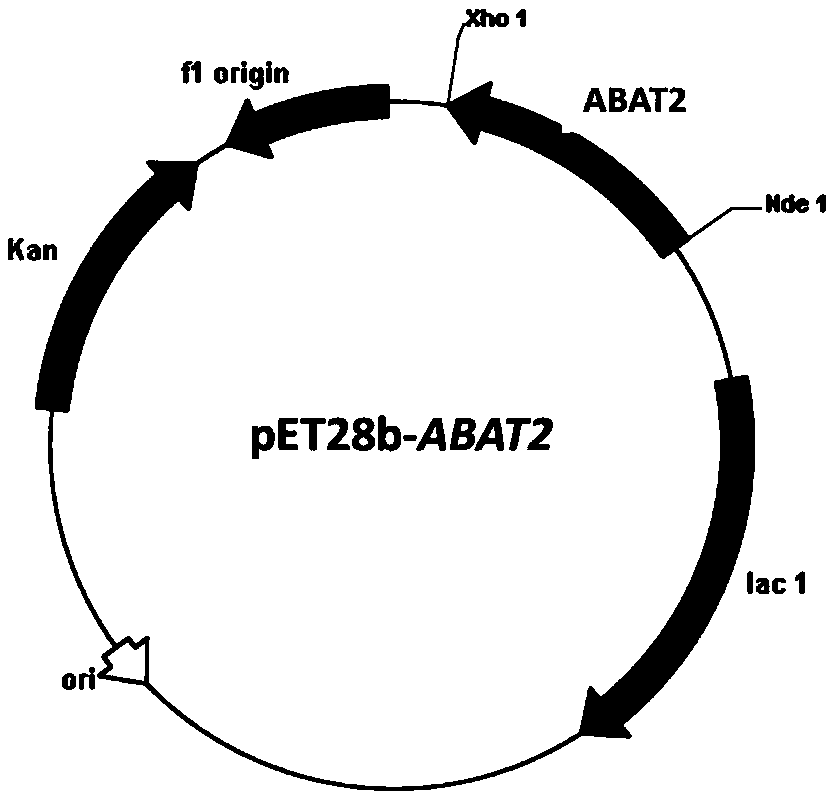
[0043] Embodiment 2: Construction of recombinant Escherichia coli BL21(DE3) / pET28b-ABAT2
[0044] According to the ABAT2 gene sequence design primer 3 (5'-CCG in embodiment 1 CATATG AACACCAAACAAC-3), Primer 4 (5'-TTG CTCGAG TTAAGCCTGTTTAGC-3'), and Nde I and Xho I restriction enzyme sites (underlined) were introduced into primer 3 and primer 4, respectively. Under the priming of primer 3 and primer 4, utilize high-fidelity PfuDNA polymerase to amplify, use recombinant plasmid pMD18-T-ABAT2 as template (obtained in embodiment 1), obtain ABAT2 gene sequence, utilize Nde I and Xho The amplified fragment was treated with I restriction endonuclease (TaKaRa), and the fragment was ligated with the commercial vector pET28b (Invitrogen) treated with the same restriction endonuclease using T4 DNA ligase (TaKaRa) to construct an expression Vector pET28b-ABAT2 ( figure 2 ). The constructed expression vector pET28b-ABAT2 was transformed into Escherichia coli BL21(DE3) (Invitrogen) (...
Embodiment 3
[0045] Embodiment 3: the preparation of recombinant transaminase (ABAT2) wet thalline
[0046] The recombinant Escherichia coli E.coliBL21(DE3) / pET28a-ABAT2 bacterial cell containing the expression recombinant plasmid pET28a-ABAT2 obtained in Example 2 was inoculated into LB liquid medium containing a final concentration of 50 μg / mL kanamycin resistance, 37 Cultivate at 200rpm for 12h, then inoculate with 1% (v / v) inoculum into fresh LB liquid medium containing kanamycin resistance at a final concentration of 50μg / ml, and cultivate at 37°C at 150rpm until the bacteria Body OD 600 After reaching 0.6-0.8, add IPTG with a final concentration of 0.1mM, induce culture at 28°C for 12h, centrifuge at 4°C and 8000rpm for 10min, discard the supernatant, collect the precipitate, and obtain recombinant Escherichia coli BL21( DE3) / pET28a-ABAT2 wet cells. The bacterium can be used directly as a biocatalyst or for protein purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com