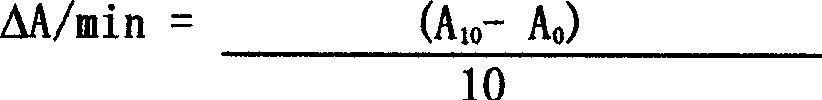Method and kit for scaling investigating angiotensin aminopherase
An angiotensin, converting enzyme technology, applied in biological testing, microbial determination/inspection, biochemical equipment and methods, etc., can solve the problems of complex experimental steps, inappropriate clinical biochemical testing, etc., and achieve the effect of improving test accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] 1. Determination of ACE standard and its activity
[0013] 1) ACE standard composition
[0014] 0-200U / L human gene recombinant ACE
[0015] 2) Determination of the activity of ACE standard
[0016] Referring to Hurst's method, add 0.1ml distilled water and 0.1ml ACE standard (0-200U / L) to a test tube containing 0.2ml incubation buffer (200mM boric acid, 2M sodium chloride pH 8.3), and incubate at 37°C for 5 minutes. The reaction was initiated by adding 0.1 ml of 20 mM HHL substrate solution for 15 minutes. Add 0.5ml of 1M hydrochloric acid (HCl) solution to terminate the reaction, and add 0.5ml of 1M sodium hydroxide (NaOH) after 30 seconds to neutralize the reaction. Add 2ml of 200mM potassium phosphate diluent pH 8.3, followed by 1.5ml of chromogenic solution (3% cyanuric acid chloride dissolved in 1,4-dioxane), mix well, let stand for 5 minutes, mix well again, and centrifuge at 3000 rpm 10 minutes. The supernatant was taken to quantitatively measure the absorb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com